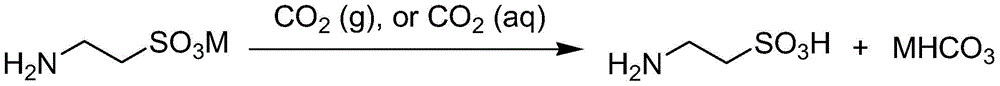Method for preparing taurine and co-producing bicarbonate
A technology of bicarbonate and taurine, applied in the preparation of alkali metal carbonate, ammonium carbonate/acid carbonate, sulfonic acid, etc. Corrosion equipment and pipelines, etc., to simplify the production process and process, reduce production costs, and solve environmental problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
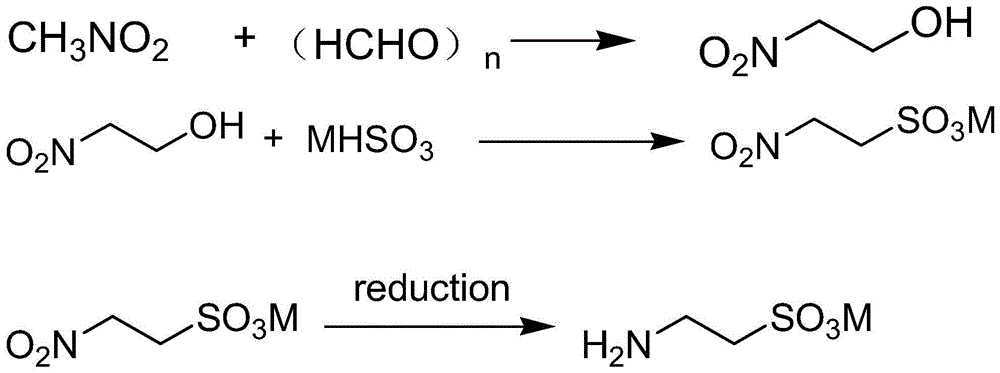
[0039] The synthesis of embodiment 12-nitroethanol
[0040] Start stirring, at room temperature, add nitromethane (550Kg, 9Kmol, 486L), ethanol 200L, paraformaldehyde (90Kg, 2.81Kmol), add potassium carbonate (1.5Kg, 10.9mol) in 1000L reactor, continue stirring 4h, and then heated to reflux for 3h. Unreacted nitromethane and solvent ethanol were recovered by distillation under reduced pressure to obtain 971.1 Kg of a light yellow oil with a yield of 96%, which was directly carried out to the next step reaction without purification.
Embodiment 2
[0041] The synthetic method of embodiment 2 ammonium aminoethylsulfonate
[0042] At -10°C, add 1200L of 10% ammonia water to the 3000L reaction kettle, feed sulfur dioxide gas until the pH value is 5.8, then add the light yellow oil containing 2-nitroethanol obtained in Example 1, stir for 15h, add Ni180g, feed hydrogen, wait for reaction to complete, separate water layer and catalyst Ni, obtain the aqueous solution of ammonium aminoethylsulfonate.
Embodiment 3
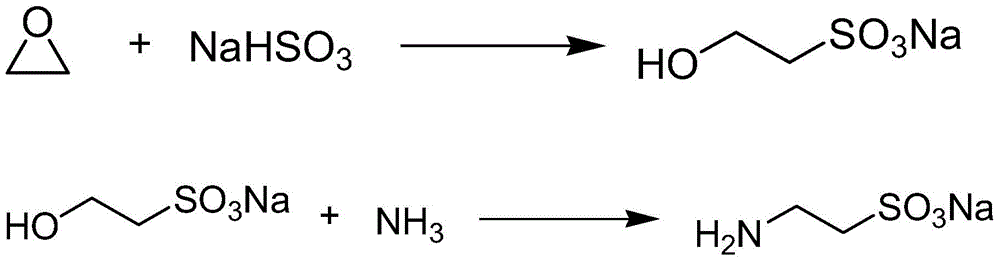
[0043] The synthetic method of embodiment 3 sodium aminoethylsulfonate
[0044]Add the sodium bisulfite solution into the hydroxylation tank, start the circulation pump, add liquid caustic soda to adjust the pH of the reaction solution, heat up to 70°C, add ethylene oxide at a certain flow rate, after the reaction is completed, cool down to 20°C, and add a certain amount of liquid caustic , add a certain amount of hydroxylated liquid to the ammonia absorption tank for absorption, stop the absorption for a certain period of time, start the high-pressure pump, and send the ammonia absorption liquid to the synthesis tower through the heater. The high temperature and high pressure in the synthesis tower, the material enters the flash evaporator through the pressure reducing valve. Sodium taurine is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com