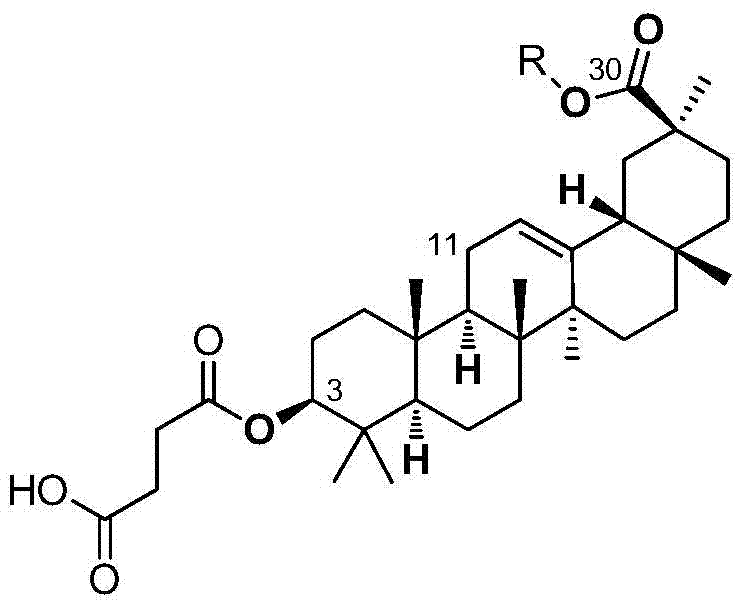
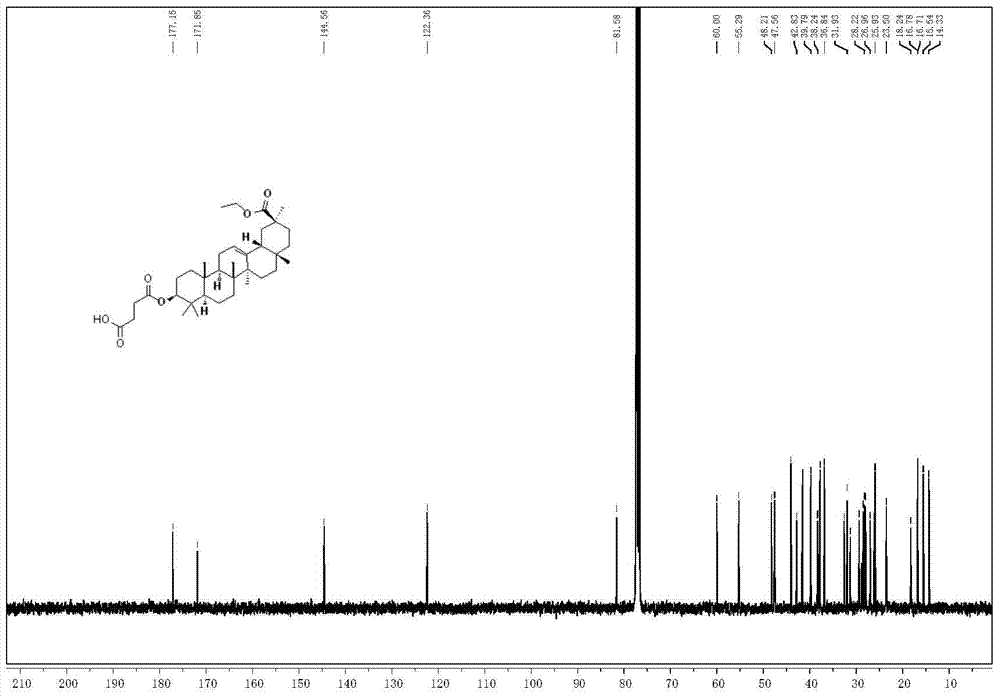
Pentacyclic triterpenoids and their use as inhibitors of human intestinal carboxylesterase
A technology of pentacyclic triterpenoids and human intestinal carboxylate, applied in the field of biomedicine, can solve the problems of affecting bioavailability, not relieving delayed diarrhea, reducing drug dosage, etc., achieving high safety and simple synthesis process OK, good security effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of (3-carboxypropionyl)-11-deoxy-glycyrrhetinic acid-30-carboxylate
[0038] (1) Synthesis of compound 1 (11-deoxy-glycyrrhetinic acid)
[0039] At room temperature, add 18β-glycyrrhetinic acid (235.3mg, 0.5mmol) and zinc powder (526.5mg, 16.2mmol) into 1,4-dioxane (9mL) solution, cool the reaction system to 8-12°C, Concentrated hydrochloric acid (1.45 mL) was slowly added dropwise, and the reaction was maintained at 8-12°C after addition, and the reaction was monitored by thin-plate chromatography (TLC). After the reaction was complete, evaporate the solvent under reduced pressure, add water (25mL), extract with dichloromethane three times (30mL×3), combine the organic phases and wash with saturated sodium chloride solution (20mL×1), dry over anhydrous sodium sulfate, and evaporate the solvent , column chromatography of the crude product (petroleum ether / ethyl acetate=20 / 1-5 / 1) gave compound 1 as a white solid with a yield of 65-75%.
[0040] (2) Synthesis ...
Embodiment 2
[0047] Quantitative evaluation of (3-carboxypropionyl)-11-deoxy-glycyrrhetinic acid-30-carboxylate for the inhibition of carboxylesterase 2
[0048] Using the hydrolytic metabolism of fluorescein diacetate as a probe reaction, the IC of inhibition of carboxylesterase 2 by glycyrrhetinic acid and its derivatives was determined by means of human liver microsome in vitro incubation system 50 :
[0049] a. 200 microliters of in vitro metabolic reaction system, containing phosphate buffer solution with a pH of 7.4, the concentration of human liver microsomal protein is 2 μg / ml, and the final concentration of inhibitors is in the range of 0.001 μM-100 μM. Shake pre-incubation at 37°C 10 minutes;
[0050] b. Add the substrate (final concentration 10 μM) to the reaction system to initiate the reaction; after reacting at 37°C for 30 minutes, add 200 μl of acetonitrile, shake vigorously, and terminate the reaction;
[0051] c. Using a high-speed refrigerated centrifuge, under the cond...
Embodiment 3
[0053] Quantitative evaluation of the inhibitory ability of (3-carboxypropionyl)-11-deoxy-glycyrrhetinic acid-30-carboxylate on carboxylesterase 1
[0054] Using the hydrolytic metabolism of fluorescein diacetate as a probe reaction, the IC of inhibition of carboxylesterase 1 by glycyrrhetinic acid and its derivatives was determined by means of human liver microsome incubation system in vitro 50 :
[0055] a. In 200 microliters of in vitro metabolic reaction system, containing phosphate buffer solution with a pH of 7.4, the concentration of human liver microsomal protein is 2 μg / ml, and the final concentration of inhibitors is in the range of 0.001 μM-120 μM. Shake pre-incubation at 37°C 10 minutes;
[0056] b. Add the substrate (final concentration 10 μM) to the reaction system to initiate the reaction; after reacting at 37°C for 30 minutes, add 200 μl of acetonitrile, shake vigorously, and terminate the reaction;
[0057] c. Using a high-speed refrigerated centrifuge, unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com