A temperature-controllable oxide thermoelectric material, a preparing method thereof and applications of the oxide thermoelectric material
A technology of thermoelectric materials and oxides, which is applied in the field of temperature-controllable oxide thermoelectric materials and its preparation, can solve the problems of unsatisfactory thermoelectric performance of carbon nanotubes, low Seebeck coefficient of carbon nanotubes, high thermal conductivity, etc. The effect of short, low cost and high Seebeck coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
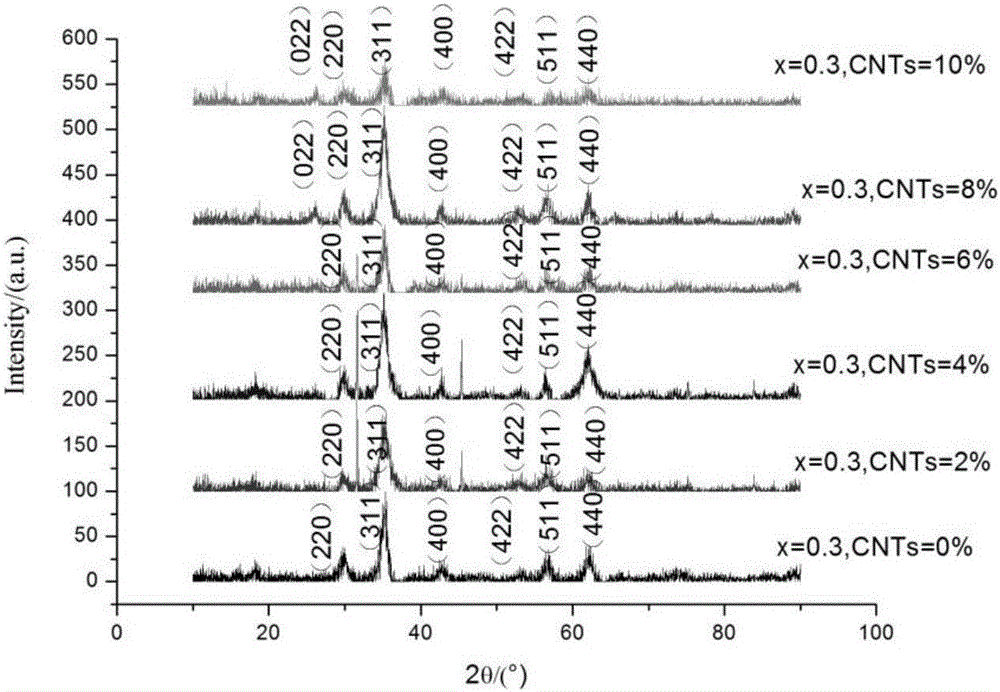
[0039] 2.9630g MnCl 2 .4H 2 O, 0.8744g ZnCl 2 , 11.5619g FeCl 3 .6H 2 O to make a metal chloride salt solution with a total metal ion concentration of 1mol / L; weigh 6.8438g NaOH to make a 1mol / L solution and add it to form Mn 0.7 Zn 0.3 Fe 2 o 4 The carbon nanotubes of 0wt%, 2wt%, 4wt%, 6wt%, 8wt%, 10wt% of mass; NaOH solution is heated up to 80 ℃, feeds high-purity nitrogen, makes NaOH solution be in high-purity nitrogen atmosphere, then The chloride salt solution was slowly added dropwise into the continuously stirring NaOH solution, and then the mixed solution was heated to 100° C. in a high-purity nitrogen atmosphere, and reacted in a high-purity nitrogen atmosphere for 1 hour. After the reaction was completed, the suspension was allowed to stand at room temperature for 5 hours, and then the precipitate was washed to neutrality with deionized water and ethanol during suction filtration, and vacuum-dried at 80°C for 10 hours to obtain 0wt%, 2wt%, 4wt%, 6wt%, 8wt%, 1...
Embodiment 2
[0043] 2.9630g MnCl 2 .4H 2 O, 0.8744g ZnCl2 , 11.5619g FeCl 3 .6H 2 O to make a metal chloride salt solution with a total metal ion concentration of 1mol / L; weigh 6.8438g NaOH to make a 1mol / L solution and add it to form Mn 0.7 Zn 0.3 Fe 2 o 4 2wt% carbon nanotubes by mass; the NaOH solution is heated up to 80° C., and high-purity nitrogen gas is passed into it, so that the NaOH solution is in a high-purity nitrogen atmosphere, and then the chloride salt solution is slowly added dropwise to the continuously stirring NaOH solution, Then the mixed solution was heated to 100° C. in a high-purity nitrogen atmosphere, and reacted in a high-purity nitrogen atmosphere for 1 hour. After the reaction was completed, the suspension was allowed to stand at room temperature for 5 hours, and then the precipitate was washed with deionized water and ethanol until neutral during suction filtration, and dried in vacuum at 80°C for 10 hours to obtain carbon nanotube-Mn 0.7 Zn 0.3 Fe 2 ...
Embodiment 3
[0047] The preparation of the powder is the same as in Example 2. Put the obtained composite powder into a graphite mold for spark plasma sintering. The heating rate is 100°C / min, and the final temperature is 700°C for 10min, and the pressure is 60MPa to obtain the carbon nanotube-Mn 0.7 Zn 0.3 Fe 2 o 4 composite material. The thermoelectric properties of the samples were tested at room temperature to 700°C.
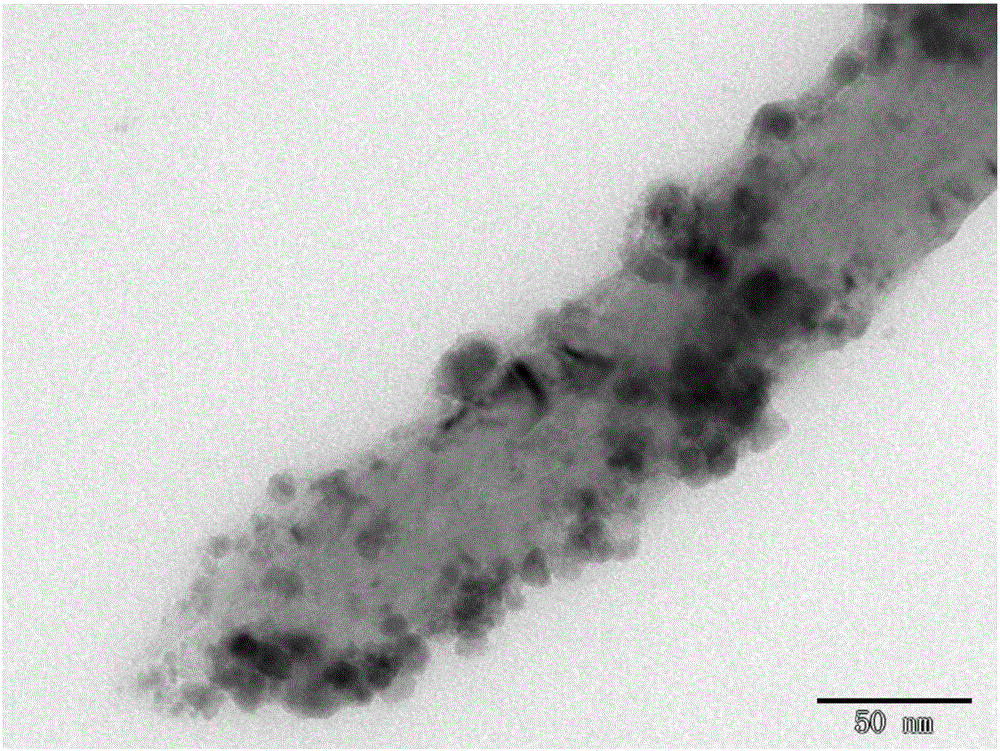
[0048] The scanning electron microscope picture of the sample prepared in this example is shown in Fig. 4(b), and it can be seen from the figure that the grain size of the sample is about 200nm.
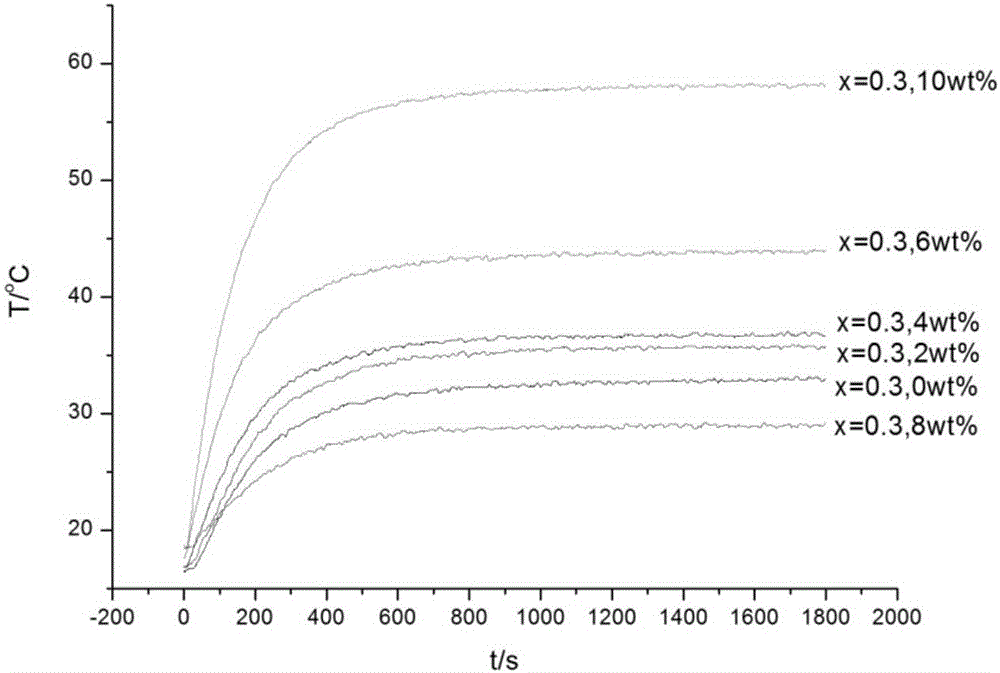
[0049] The thermoelectric performance of the sample prepared in this example varies with temperature curve as Figure 5-9 As shown, it can be seen from the figure that the conductivity of the composite material at 700°C is 963.419S / m, the Seebeck coefficient is -184.271μV / K, and the power factor is 3.271×10 -5 W / mK 2 , thermal conductivity=1.583W / mK, ZT=0.02011.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com