Thermally stable lipase as well as preparation method and applications thereof
A technology of lipase and thermostabilization, applied in the field of enzyme engineering, can solve problems such as the design of protein dynamic stability and the lack of a deep understanding of protein unfolding kinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
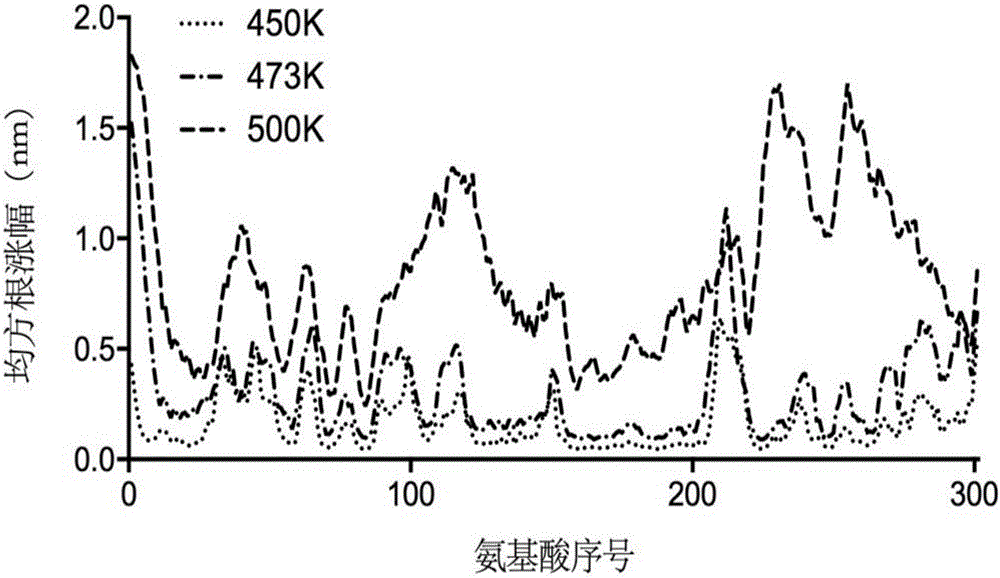
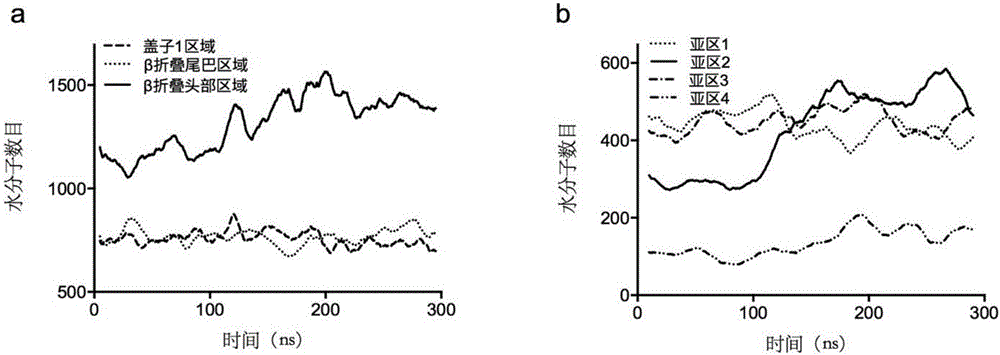
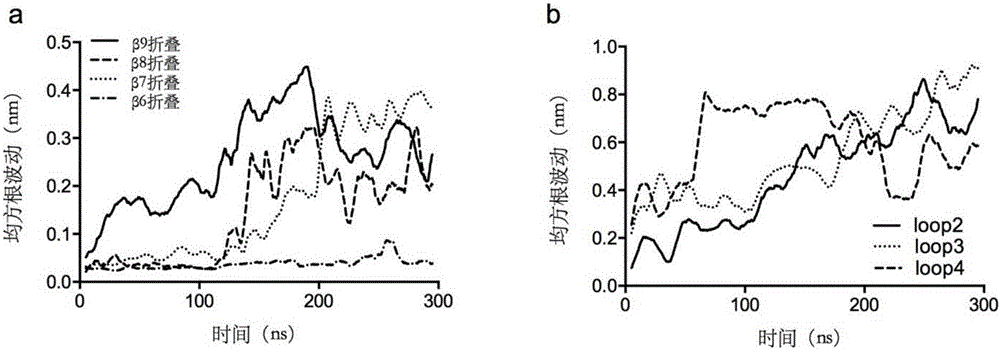
[0063] Example 1 Establishment of unfolding model of Yarrowia lipolytica lipase 2 and screening of disulfide bonds.
[0064] (1) Search and download the crystal structure of Lip2 (PDB ID: 3O0D) from the RCSB PDB crystal database with an accuracy of The crystal is a heptamer, and each polymer has different degrees of missing atoms. Therefore, the complete A-chain of the skeleton atoms is selected as the initial model, and the missing side chain amino acids of the A-chain are repaired using the Swiss-pdb Viewer software, and Disulfide is used to byDesign software, the Cys120-Cys123 disulfide bond in the A chain is reconstructed, and all the crystal water of the A chain is retained in the simulation. Except for the HIS289 in the center of the triplet, which uses the HID protonation state, the rest of the histidines are set to the default pH of Gromacs The protonation state of =7.00 obtains the pretreatment model;
[0065] (2) Use the GROMACS 5.04 software to carry out three-d...
Embodiment 2
[0101] The construction of embodiment 2 lipase mutant expression plasmid
[0102] Using the Yarrowia lipolytica lipase 2 sequence (Genbank ID: AJ012632.1) as the target fragment, with EcoRI and NotI as the restriction enzyme sites, the whole gene was synthesized and constructed pPICZαA-Lip2, through two consecutive reverse The mutation PCR method introduces disulfide bond mutation, and the primers used for point mutation are shown in Table 2.
[0103] Table 2 Summary of mutation primers
[0104]
[0105]
[0106] Note: The bold slash is the mutation site
[0107] The PCR amplification conditions are: 94°C for 2min; 94°C for 10s, 66°C for 30s, 68°C for 5min, 10 cycles. The reaction system is shown in Table 3 below.
[0108] Table 3 PCR reaction system
[0109]
[0110] The amplified product was digested with DnpI enzyme to digest the template, and after agarose gel electrophoresis to detect the size of the mutant band, it was ligated and circularized overnight with...
Embodiment 3
[0111]Example 3: Electrotransformation of Pichia pastoris with linearized plasmid, screening of transformants and screening of enzyme production
[0112] After the positive transformants with correct sequencing were expanded overnight in LLB liquid medium, the plasmid was extracted, linearized with PmeI, purified and recovered, and a total of 5 μg of the plasmid linearized product was mixed with X33 Pichia pastoris for electroporation transformation. Competent preparation of Pichia pastoris refers to the operation manual of Invitrogen Company. The electroporation program was set according to the parameters recommended by Bio-Rad.
[0113] Add 1 mL of 1mol / L sorbitol solution immediately after electroporation, incubate and recover the bacterial solution at 30°C for 1 hour, and spread it evenly on the YPDS+Zeocin (Zeocin concentration is 200 μg / ml) resistance plate for screening; after culturing for 3 days, put The grown single colony was picked on the BMMY-rhodamine B plate fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com