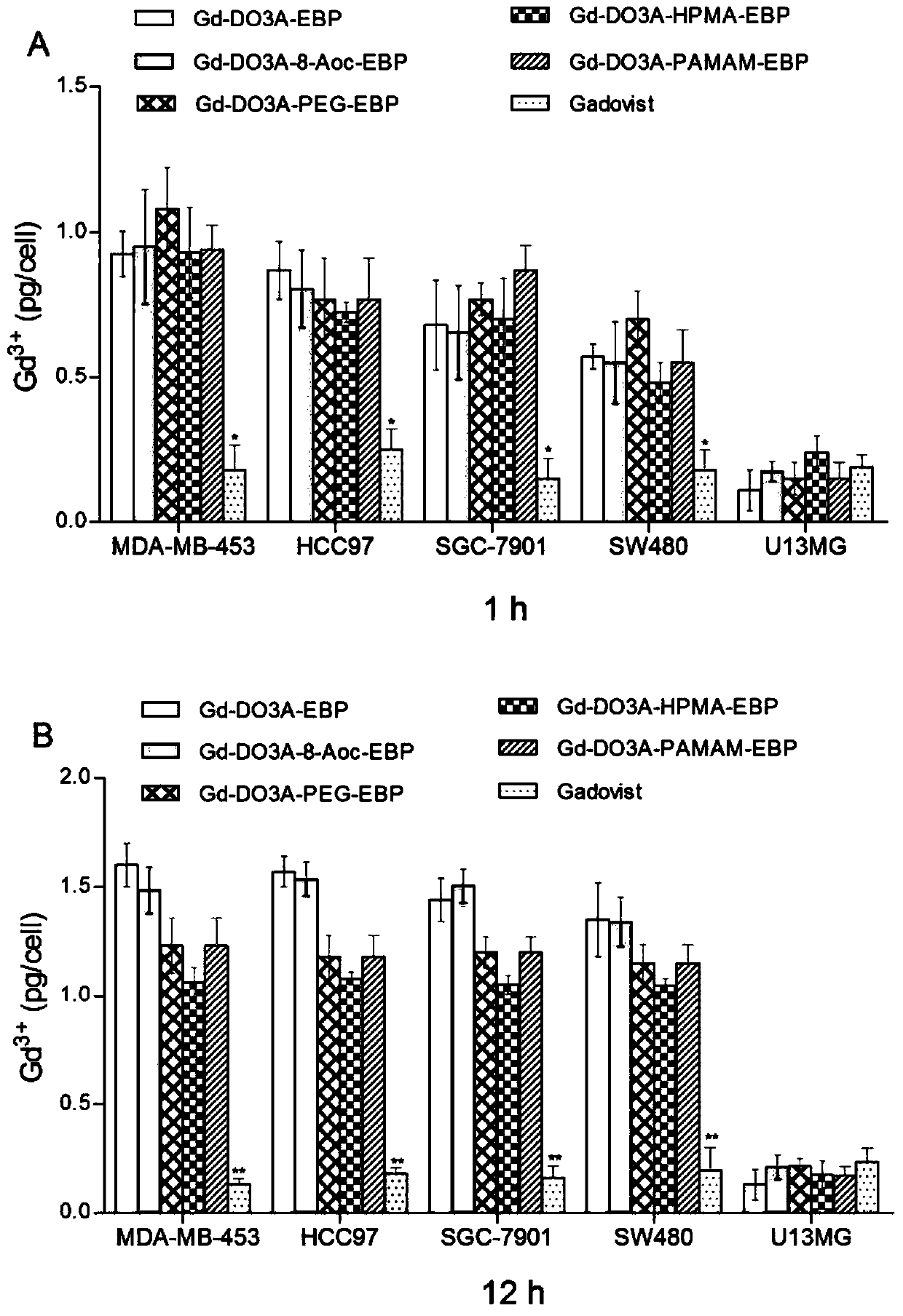
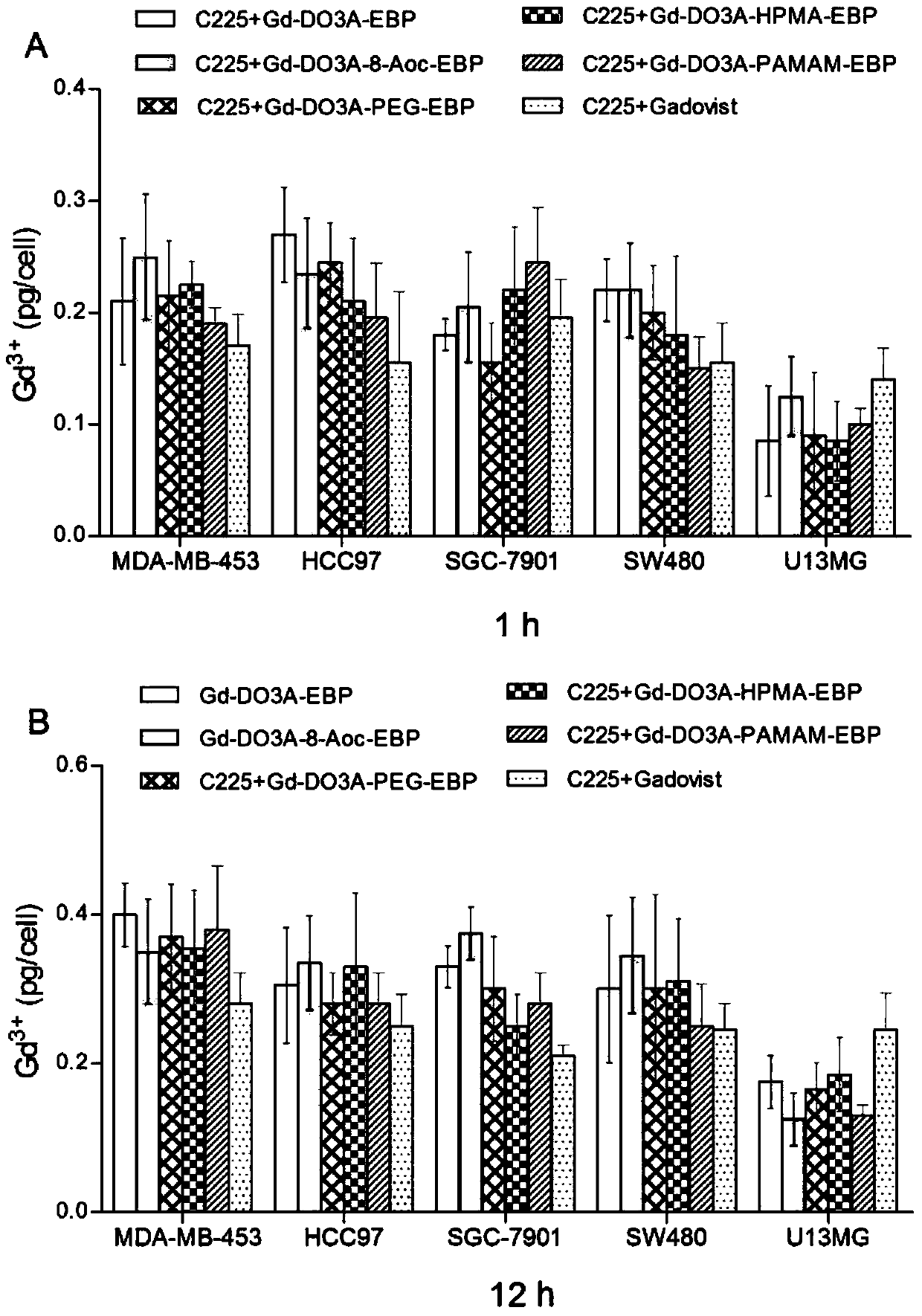
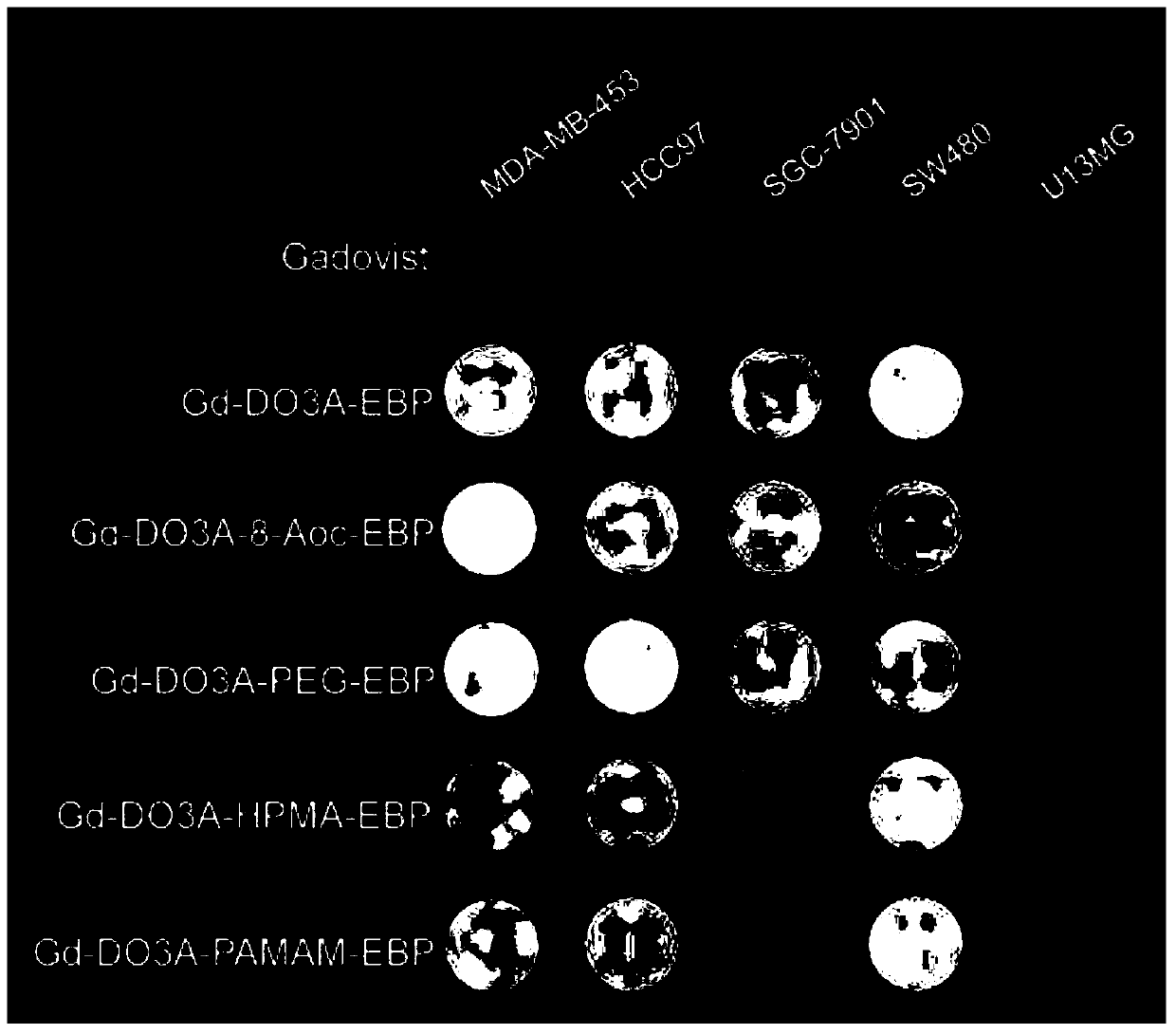
A kind of tumor-targeted MRI contrast agent and preparation method thereof
A tumor-targeted, contrast agent technology used in the field of medical imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Synthesis of DO3A-tris(t-Bu)-EN (DO3A-tri-tert-butylethylenediamine)
[0025] 4.0g DO3A-tris(t-Bu) (DO3A-tri-tert-butyl ester) and 4.3g K 2 CO 3 Dissolve in 40mL of acetonitrile, stir for 1h, add 0.65mL of bromoacetonitrile, continue stirring for 8h, after the reaction is complete, filter the reactant through a G4 sintered funnel, remove the solvent by rotary evaporation under reduced pressure, and obtain a gel by silica gel column chromatography . Then, the above colloidal intermediate product was dissolved with 30 mL of 7N ammonia / methanol, and after adding 1.5 g of Raney nickel (Ra-Ni) catalyst, the mixture was heated at room temperature and 50 psi H 2 After the reaction was completed, the reactant was filtered through a G4 sintered funnel, the solvent was removed by rotary evaporation under reduced pressure, and DO3A-tris(t-Bu)-EN was obtained by silica gel column chromatography.
Embodiment 2
[0026] Embodiment 2: the synthesis of Gd-DO3A-EBP
[0027] Dissolve 0.88g DO3A-tris(t-Bu)-EN, 0.8g EBP and 0.33g 1-hydroxybenzotriazole (HOBt) in 5mL DMF, add 0.35mL N-methylmorpholine (NMM), After stirring at 0°C for 15 minutes, add 0.23 mg of N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide (EDC), continue to stir overnight, and remove the solvent by rotary evaporation under reduced pressure after the reaction is complete. HPLC Purified and freeze-dried to obtain DO3A-EBP as a white powder. DO3A-tris(t-Bu)-SH and EBP were dissolved in DMSO, hydrogen peroxide was added, and stirred at 0°C for 2 hours to obtain DO3A-EBP linked by disulfide bonds.
[0028] Weigh 96mg DO3A-EBP, dissolve in 10mL water, add excess Gd(OAc) 3 , add 0.01N NaOH dropwise to adjust the pH to 5.5-6.0, stir the reaction at 50°C for 48h, and remove the free Gd with dipotassium ethylenediaminetetraacetic acid (EDTA) 3+ , made of Gd-DO3A-EBP.
Embodiment 3
[0029] Example 3: Synthesis of Gd-DO3A-8-Aoc-EBP
[0030]Dissolve 0.5g EBP in 40mL DMF, add 0.2g BOP reagent and 0.1g HOBt, stir at room temperature, add 0.6mL N,N-diisopropylethylamine (DIPEA) dropwise, add 0.32g suberic acid, Stir at low temperature for 1 hour, remove the solvent under vacuum, extract with 10 mL of ethyl acetate for crystallization, and purify by HPLC to obtain hemisuberic anhydride-EBP. Dissolve 0.28g DO3A-tris(t-Bu)-EN, 0.16g hemisuberic anhydride-EBP and 0.33g HOBt in 5mL DMF, add 0.35mL NMM, stir at 0°C for 15min, then add 0.1mg EDC, continue stirring to make Overnight, after the reaction was complete, the solvent was removed by rotary evaporation under reduced pressure, purified by HPLC, and freeze-dried to obtain DO3A-8-Aoc-EBP. Weigh 96mg DO3A-8-Aoc-EBP, dissolve in 10mL water, add excess Gd(OAc) 3 , add dropwise 0.01NNaOH to adjust the pH to 5.5-6.0, stir the reaction at 50°C for 48h, remove free Gd with dipotassium EDTA 3+ , to make Gd-DO3A-8-Aoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com