Hydrogel sustained-release carrier for oral drugs as well as preparation method and application of hydrogel sustained-release carrier
A slow-release carrier and hydrogel technology, which can be used in drug combination, drug delivery, and pharmaceutical formulations, etc. It can solve the problems of easily residual initiators and cross-linking agents, calcium alginate is not easily soluble in water, and poor biocompatibility , to achieve excellent biocompatibility and degradation performance, excellent tissue compatibility, and optimized performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
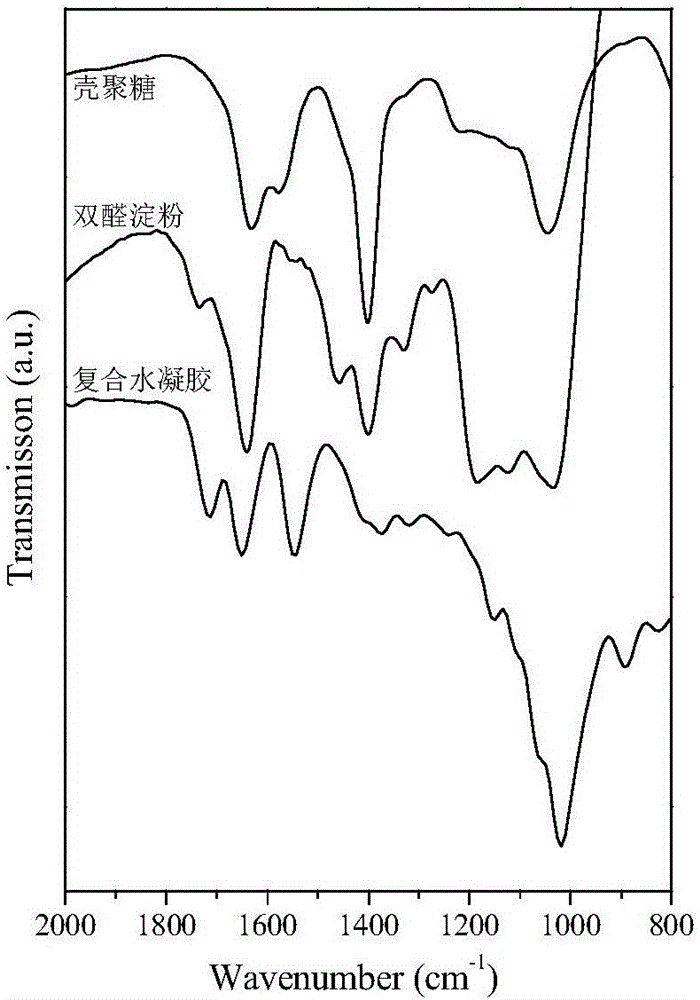
[0027] The degree of deacetylation of the chitosan is greater than 85%, and the purity of the dialdehyde starch, gelatin and polyvinyl alcohol all reach the pharmaceutical standard. 2g chitosan, 2g dialdehyde starch, 2g gelatin, and 2g polyvinyl alcohol were dissolved in 50g deionized water respectively, and the prepared chitosan solution, dialdehyde starch solution, gelatin solution, and polyvinyl alcohol solution were mixed in 5: The volume ratio of 0.8:3:0.5 is mixed evenly. Put the prepared solution into an acidic aqueous solution with a pH of 3.0 to catalyze cross-linking, and obtain a hydrogel after standing for 5 minutes, soak it in deionized water several times to neutrality, and obtain a finished hydrogel, freeze-dry it, and The dried hydrogel was analyzed by infrared spectroscopy (results are attached figure 1 ). It can be seen from the figure that at 1450-1670cm -1 The vibrational peaks of , confirm the presence of Schiff bases (i.e., imine bonds), proving the fo...
Embodiment 2
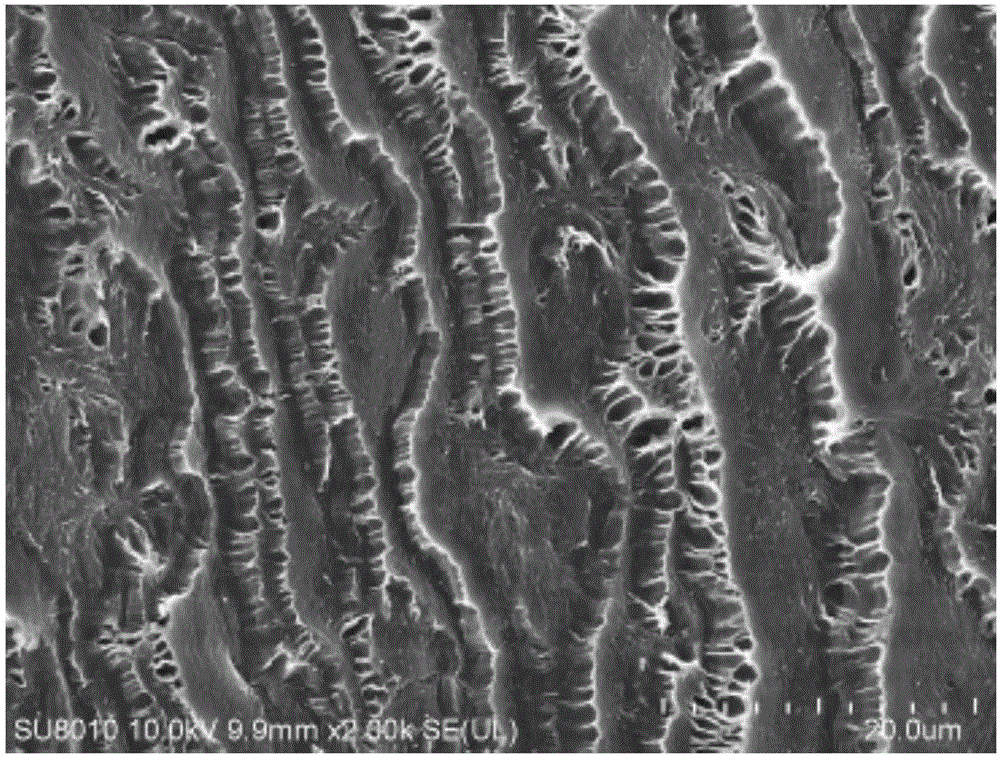
[0029] 2g chitosan, 2g dialdehyde starch, 2g gelatin, and 2g polyvinyl alcohol were dissolved in 50g deionized water respectively, and the prepared chitosan solution, dialdehyde starch solution, gelatin solution, and polyvinyl alcohol solution were mixed in 5: The volume ratio of 1.2:3.5:0.6 is mixed evenly. Add the prepared solution to an acidic solution with a pH of 4.0 to catalyze it. After standing for 5 minutes, a hydrogel is obtained. Soak it in deionized water until neutral to obtain a finished hydrogel. After freeze-drying, it will be brittle after cooling with liquid nitrogen. , exposed the section, and analyzed the hydrogel section by scanning electron microscopy (results are shown in the attached figure 2 ). It can be seen from the figure that the cross-section of the hydrogel is a multilayer structure with a large number of cavities, which proves that the hydrogel has excellent water absorption and swelling properties.
Embodiment 3
[0031] 2g chitosan, 2g dialdehyde starch, 2g gelatin, and 2g polyvinyl alcohol were dissolved in 50g deionized water respectively, and the prepared chitosan solution, dialdehyde starch solution, gelatin solution, and polyvinyl alcohol solution were mixed in 5: The volume ratio of 1.6:4:0.7 is evenly mixed. The prepared solution is added to an acidic solution with a pH of 3 for catalysis, and the hydrogel is obtained after standing for 5 minutes, soaked in deionized water until neutral, and the finished hydrogel is obtained. Freeze-drying, scanning electron microscopy on the surface of the hydrogel (results are attached image 3 ). It can be seen from the figure that the microscopic surface of the hydrogel surface is smooth, and the fractures are relatively regular.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com