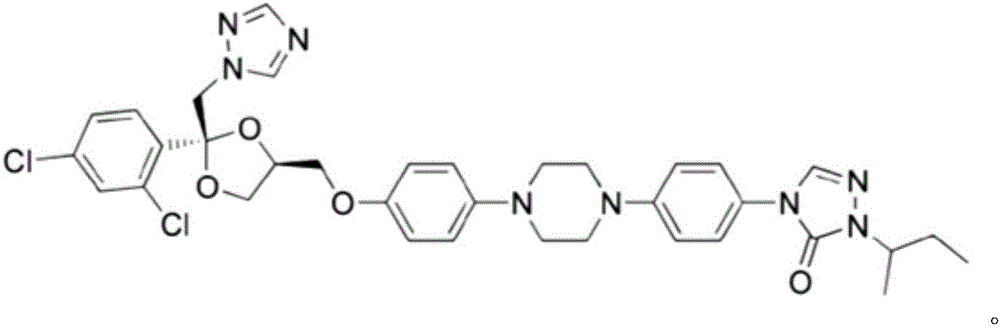
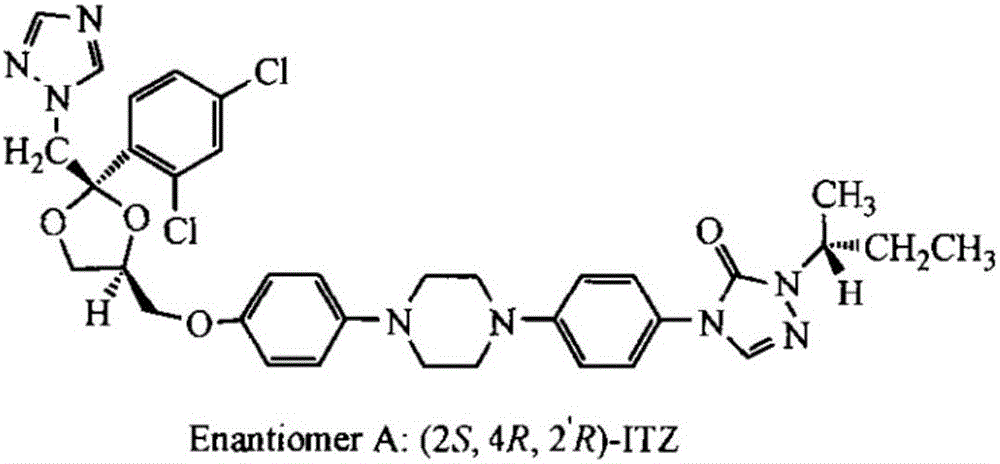
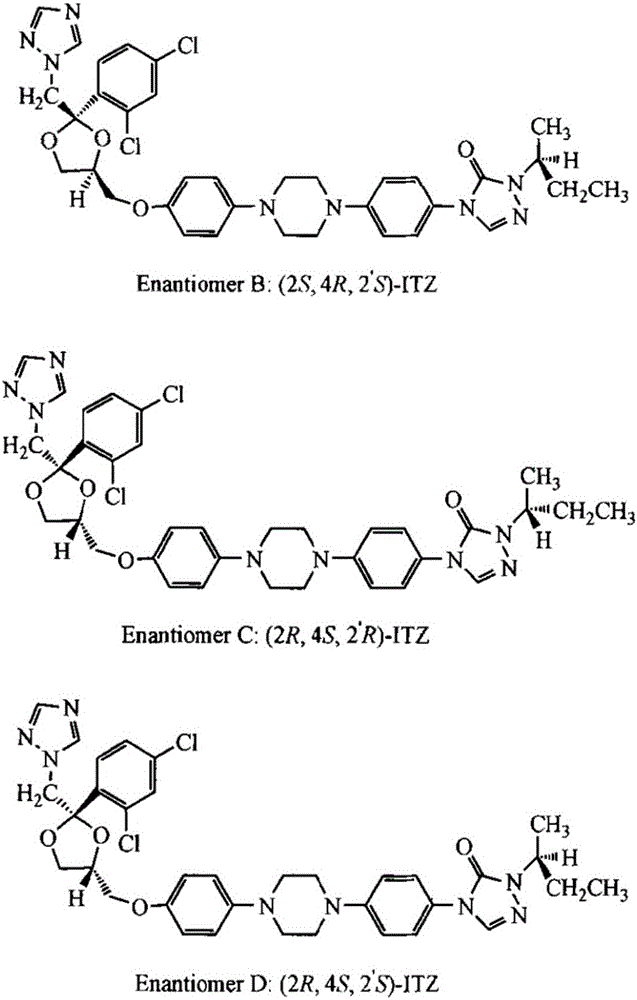
Prepartion method of itraconazole
An itraconazole and compound technology, applied in the field of preparation of itraconazole, can solve the problems of affecting the internal quality control of itraconazole products, the price of cis-bromoester is relatively expensive, the process operation is complicated and tedious, and the The effect of high reaction selectivity and purity, environmental friendliness and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0043] Embodiment 1-1: the synthesis of trityl-glycidyl ether (1)
[0044]Trityl chloride (139.4g, 0.5mol) was dissolved in 5000mL of dichloromethane, 4-diaminopyridine (DMAP, 3g, 25mmol) and triethylamine (100mL) were added, and (±)-epoxypropylene was dropped under stirring Dichloromethane solution (500mL) of alcohol (37g, 0.5mol) was reacted at room temperature for 3 hours; a large amount of white solid was precipitated, the solid was filtered off, the solution was washed with saturated sodium chloride solution (300mL), dried over anhydrous sodium sulfate, and concentrated Recrystallized from absolute ethanol and dried to obtain a white solid (109.6 g, 1), with a yield of 69.3%.
Embodiment 1-2
[0045] Embodiment 1-2: the synthesis of trityl-glycidyl ether (1)
[0046] Trityl chloride (153.3g, 0.55mol) was dissolved in 5000mL chloroform, 4-diaminopyridine (DMAP, 3g, 25mmol) and triethylamine (120mL) were added, and (±)-glycidyl alcohol ( 37g, 0.5mol) in chloroform solution (500mL), reacted at room temperature for 5 hours; a large amount of white solid was precipitated, the solid was filtered out, the solution was washed with saturated sodium chloride solution (300mL), dried over anhydrous sodium sulfate, concentrated and then washed with absolute ethanol Recrystallized and dried to obtain a white solid (119.0 g, 1), with a yield of 75.2%.
Embodiment 2-1
[0047] Embodiment 2-1: Preparation of compound 2
[0048] Sodium hydride (50% in mineral oil, 0.7 mol) was washed twice with 1 L of hexane, then dried under nitrogen. Dry dimethylformamide (0.5 L) was added. Then, keeping the temperature below 50°C, benzyl alcohol (140 mL) was added dropwise at a certain rate, and the dropwise addition was completed within 2 hours. Compound 1 (110.7g, 0.35mol) was added dropwise for 0.5 hours, cooled to keep the temperature below 40°C, stirred at 20°C for 16 hours, and then stirred at 50°C for 2.5 hours. Evaporate under reduced pressure to remove dimethylformamide, dissolve the oily residue with 1L of ether, and use 0.5L of water, 0.5L of 2% hydrochloric acid solution, 0.5L of 1% sodium bicarbonate solution, and 0.35 L was washed with brine, dried over anhydrous sodium sulfate, and concentrated to give a brown oil (compound 2, 104.5 g), with a yield of 70.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com