Lithium battery negative electrode material and preparation method thereof
A negative electrode material, lithium battery technology, applied in battery electrodes, secondary batteries, nanotechnology for materials and surface science, etc., to shorten the preparation period, avoid electrode crushing, and improve cycle stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
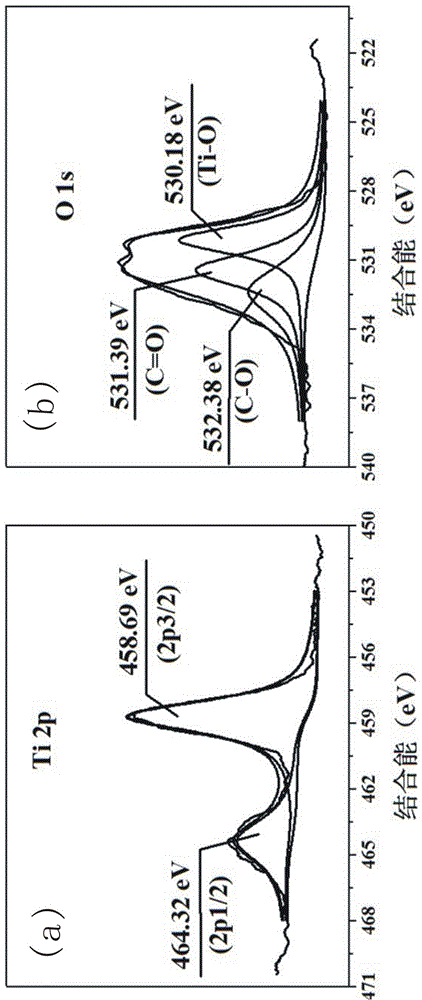
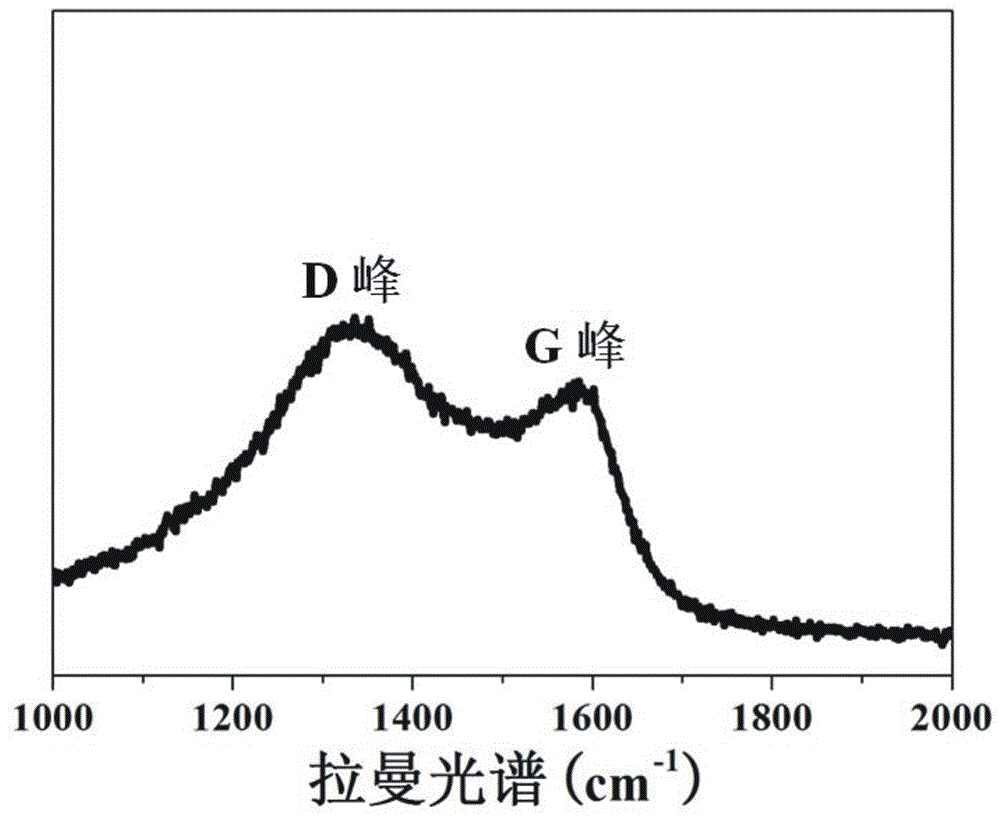
[0044] Stir and mix 5 parts of collagen fibers and 400 parts of deionized water evenly, adjust the pH of the system to 1.5-2.0, add titanium sulfate and stir for 4 h, wherein Ti 4+ The content is 40% of the collagen fiber mass. Subsequently, the pH of the system was adjusted to 5.0–5.5, and the temperature was raised to 45 ºC to continue the reaction for 6 h. After the reaction was completed, it was filtered, washed, and dried to obtain collagen fiber-loaded Ti (CF@Ti); the obtained CF@Ti was added to Stir and mix 400 parts of deionized water evenly, then add 0.1 part of bayberry tannin, and stir and react at room temperature for 4 h, filter, wash, and dry; the obtained solid powder is carbonized at high temperature for 3 h under vacuum conditions, and the carbonization temperature is 800 ºC, you can get C@TiO 2 @C.
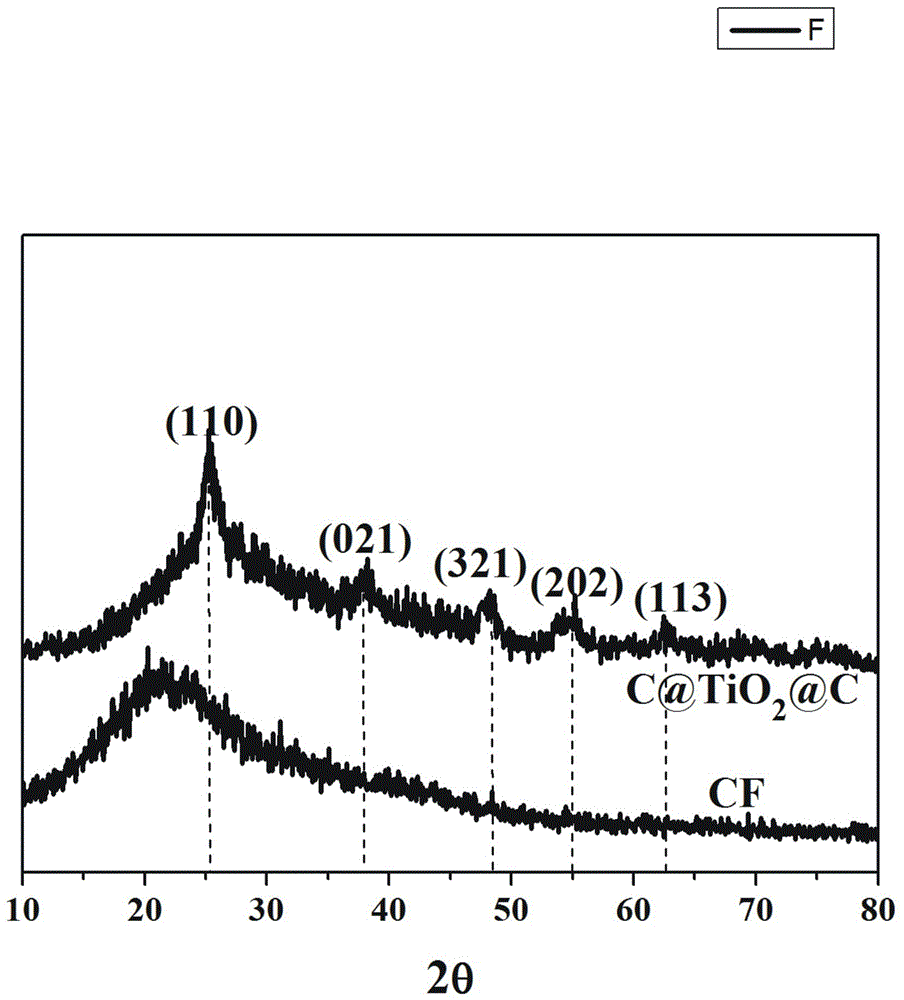
[0045] Depend on figure 1 It can be seen that C@TiO 2 @C has TiO 2 Typical (311), (400) and (511) crystal plane diffraction peaks.
[0046] Depend on fig...
Embodiment 2
[0052] Stir and mix 5 parts of collagen fibers with 350 parts of deionized water, adjust the pH of the system to 1.5~2.0, and then add SnCl 4 ·5H 2 O stirred the reaction for 4 h, where Sn 4+The dosage is 20% of the collagen fiber mass. Subsequently, the pH of the system was adjusted to 3.5-4.0, and the temperature was raised to 40 ºC to continue the reaction for 6 h. After the reaction was completed, it was filtered, washed, and dried to obtain collagen fiber-loaded Sn (CF@Sn); 0.005 parts of bayberry tannin was dissolved In 50 parts of deionized water, add 1 part of CF@Sn to the above tannin solution, and continue to stir and react at room temperature for 4 h, filter, wash, and dry; the obtained solid powder is carbonized at high temperature for 2 h under vacuum conditions , the carbonization temperature is 500 ºC, and the C@SnO 2 @C.
[0053] The resulting C@SnO 2 @C was assembled into a coin cell as the working electrode, and its cycle stability was tested on a charge...
Embodiment 3
[0055] After stirring and mixing 5 parts of collagen fibers with 350 parts of deionized water, adjust the pH of the system to 1.5~2.0, and then add SnCl 4 ·5H 2 O stirred the reaction for 4 h, where Sn 4+ The dosage is 30% of the collagen fiber mass. Subsequently, the pH of the system was adjusted to 3.5-4.0, and the temperature was raised to 40 ºC to continue the reaction for 6 h. After the reaction was completed, it was filtered, washed, and dried to obtain collagen fiber-loaded Sn (CF@Sn); 0.025 parts of myricetin was dissolved In 50 parts of deionized water, add 1 part of CF@Sn to the above tannin solution, and continue to stir and react at room temperature for 4 h, filter, wash, and dry; the obtained solid powder is carbonized at high temperature for 2 h under vacuum conditions , the carbonization temperature is 500 ºC, and the C@SnO 2 @C.
[0056] The resulting C@SnO 2 @C was assembled into a button battery as the working electrode, and the cycle performance was tes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com