Tosufloxacin tosylate dispersible tablets and preparation method thereof
A technology of tosufloxacin tosylate and dispersible tablets, which is applied in the field of medicine, can solve the problems of low bioavailability, poor capsule stability, and poor antibacterial effect, and achieve good antibacterial effect, stable properties, and good disintegration effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) At a temperature of 40°C, dissolve the crude product of tosufloxacin tosylate in a mixed solution of ethanol and water with a volume fraction of 95%, mix and stir to dissolve evenly, wherein the volume ratio of ethanol to water is 1 : 1, the mass volume ratio of tosufloxacin tosylate and ethanol and water mixed solution is 1g: 10ml.
[0040] (2) adding an appropriate amount of mass fraction to the above solution is 0.2% activated carbon, and then filters;
[0041] (3) The filtered solution is cooled to 30°C, left to stand for 15 hours, and crystallized;
[0042] (4) After the crystals are completely precipitated, they are washed and dried at 50° C. to obtain the tosufloxacin tosylate crystals.
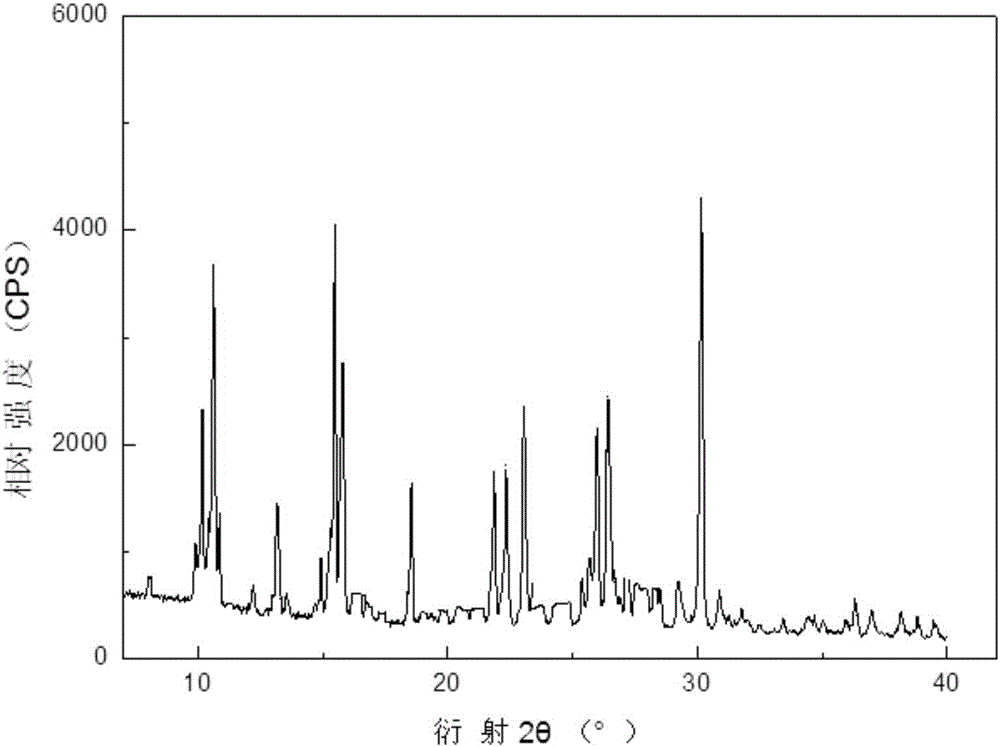
[0043] The X-ray powder diffraction spectrum that the tosufloxacin tosylate crystal that obtains uses Cu-Kα ray measurement to obtain is as follows figure 1 Shown, the X-ray powder diffraction of tosufloxacin tosylate crystal is 10.13°, 10.61°, 13.21°, 15.42°, 15.81°, 18.4...
Embodiment 2
[0045] (1) At a temperature of 45°C, dissolve the crude product of tosufloxacin tosylate in a mixed solution of ethanol and water with a volume fraction of 95%, mix and stir to dissolve evenly, wherein the volume ratio of ethanol to water is 10 : 1, the mass volume ratio of tosufloxacin tosylate and ethanol and water mixed solution is 1g: 5ml.
[0046] (2) adding an appropriate amount of mass fraction to the above solution is 0.1% activated carbon, and then filters;
[0047] (3) The filtered solution is cooled to 28°C, left to stand for 10 hours, and crystallized;
[0048] (4) After the crystals are completely precipitated, they are washed and dried at 40° C. to obtain the tosufloxacin tosylate crystals.
[0049]The X-ray powder diffraction spectrum obtained by measuring the obtained tosufloxacin tosylate crystals using Cu-Kα rays is basically consistent with that of Example 1.
Embodiment 3
[0051] (1) At a temperature of 80°C, dissolve the crude product of tosufloxacin toluenesulfonate in a mixed solution of ethanol and water with a volume fraction of 95%, mix and stir to dissolve evenly, wherein the volume ratio of ethanol to water is 5 : 1, the mass volume ratio of tosufloxacin tosylate and ethanol and water mixed solution is 1g: 3ml.
[0052] (2) adding an appropriate amount of mass fraction to the above solution is 0.3% activated carbon, and then filters;
[0053] (3) The filtered solution is cooled to 32°C, left to stand for 20 hours, and crystallized;
[0054] (4) After the crystals are completely precipitated, they are washed and dried at 60° C. to obtain the tosufloxacin tosylate crystals.
[0055] The X-ray powder diffraction spectrum obtained by measuring the obtained tosufloxacin tosylate crystals using Cu-Kα rays is basically consistent with that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com