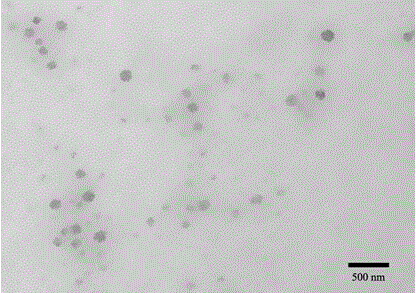
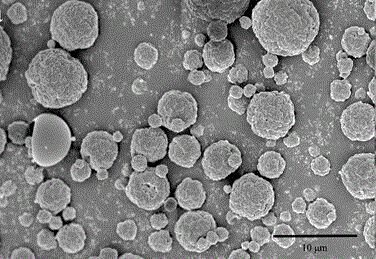
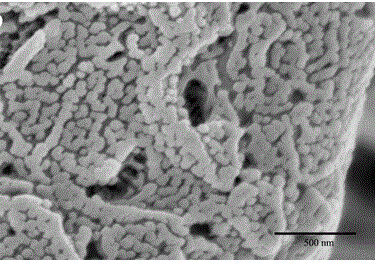
A pulmonary inhaled chitosan-based nano targeting polymer particles and its production method thereof
A technology of chitosan nanoparticles and chitosan, which is applied in the medical basic application field of biological materials, can solve the problems of easy to cause toxic reactions, poor biocompatibility, low cell phagocytosis rate, etc., and achieves increased bioavailability, biological Good compatibility and uniform particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1 A kind of chitosan-based targeted nanopolymer particles for lung inhalation
[0069] The nanopolymer particles include chitosan, sodium tripolyphosphate, cetuximab, lecithin, hydroxypropyl β-cyclodextrin and excipients;
[0070] Described chitosan, molecular weight is 1120Kda; Deacetylation degree 96%, powder form;
[0071] The phospholipid is lecithin;
[0072] The excipient is lactose;
[0073] The mass ratio of chitosan, sodium tripolyphosphate, cetuximab, lecithin, hydroxypropyl beta-cyclodextrin and lactose is 5:1:0.01:2:1:5;
[0074] The preparation method of the lung inhalation chitosan-based targeting nano-polymer particles comprises the following steps:
[0075] (1) Preparation of chitosan solution
[0076] Weigh 500 mg of chitosan, dissolve it in 250 mL of 1% acetic acid solution, and prepare a 2 mg / mL chitosan solution.
[0077] (2) Preparation of sodium tripolyphosphate solution
[0078] Weigh 100 mg of sodium tripolyphosphate, dissolve so...
Embodiment 2
[0096] Embodiment 2 A kind of chitosan-based targeting nanopolymer particles for lung inhalation
[0097] The nanopolymer particles include chitosan derivatives, sodium tripolyphosphate, cetuximab, phospholipid derivatives, hydroxypropyl β-cyclodextrin and excipients;
[0098] Described chitosan derivative, molecular weight is 1250Kda, and degree of substitution is carboxymethyl chitosan of 86%, powder form;
[0099] The phospholipid derivative is dipalmitoylphosphatidylcholine DPPC
[0100] The excipient is mannitol;
[0101] The carboxymethyl chitosan, sodium tripolyphosphate, cetuximab, DPPC, hydroxypropyl β-cyclodextrin, and mannitol mass ratio are 4:2:0.02:3:3:1;
[0102] The preparation method of the lung inhalation chitosan-based targeted nanopolymer particle comprises the following steps:
[0103] (1) Preparation of chitosan solution
[0104] Weigh 400 mg of carboxymethyl chitosan, and dissolve carboxymethyl chitosan powder with a deacetylation degree of 96% in 200...
Embodiment 3
[0122] Example 3 A kind of chitosan-based targeted nanopolymer particle for lung inhalation
[0123] The nanopolymer particles include chitosan derivatives, sodium tripolyphosphate, cetuximab, phospholipid derivatives, hydroxypropyl β-cyclodextrin and excipients;
[0124] Described chitosan derivative, molecular weight is that 1000Kda degree of substitution is the chitosan quaternary ammonium salt of 90%, powder form;
[0125] The phospholipid derivative is 1,2-palmitoylphosphatidylglycerol sodium salt DPPG
[0126] The excipient is trehalose;
[0127] The chitosan quaternary ammonium salt, sodium tripolyphosphate, cetuximab, DPPG, hydroxypropyl β-cyclodextrin, and trehalose have a mass ratio of 3:2:0.01:1:3:5;
[0128] The preparation method of the lung inhalation chitosan-based targeted nanopolymer particle comprises the following steps:
[0129] (1) Preparation of chitosan solution
[0130] Weighed 300 mg of chitosan quaternary ammonium salt, and dissolved chitosan powd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com