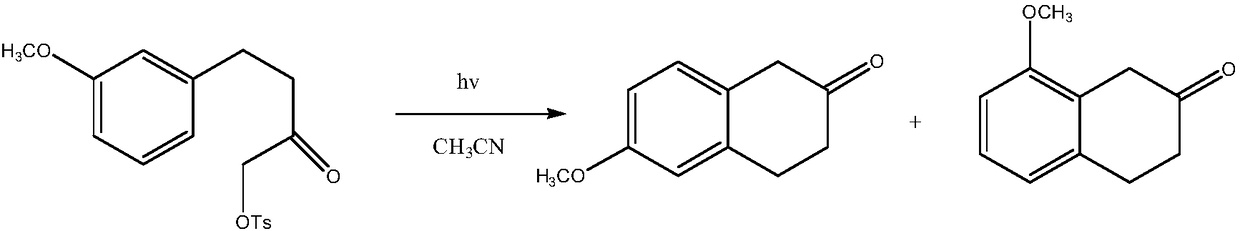
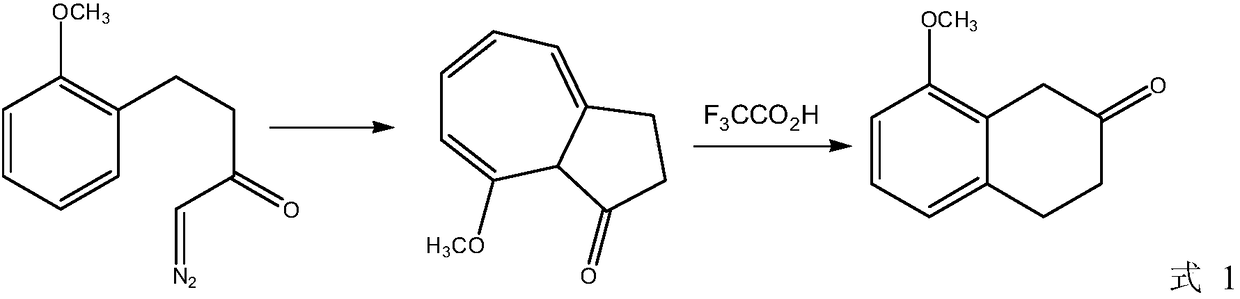
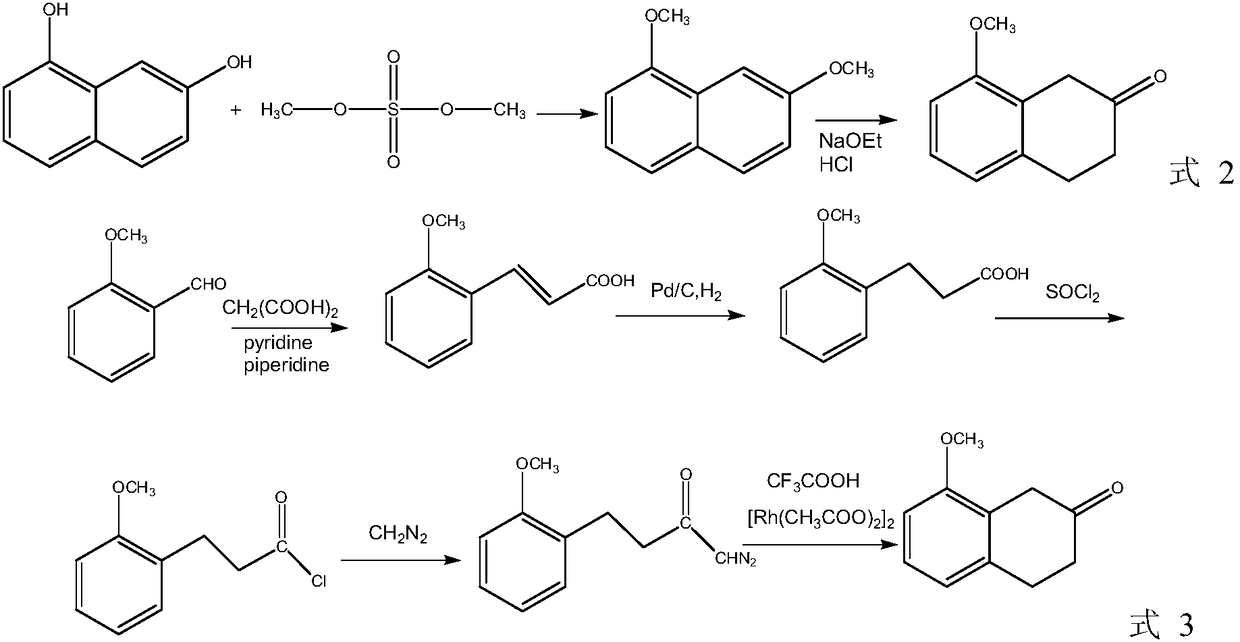
A kind of preparation method of 8-methoxy-3,4-dihydro-1h-2-naphthalenone
A technology of -1H-2-, methoxy, which is applied in the field of preparing 8-methoxy-3,4-dihydro-1H-2-naphthalene, can solve the problem of difficult extraction of nitrilase, high production cost, Using expensive catalysts and other issues to achieve the effect of mild process reaction conditions, low production costs, and good paramagnetism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]Add 2-methoxybenzonitrile (13.3g, 0.1mol) into a 100mL round-bottomed flask, add a solution of sulfuric acid (9.8g, 0.1mol) dropwise, after the dropwise addition, keep the temperature at 125-130°C for 2 hours, and react Finished, cooled to below 100°C, diluted with water, cooled to below 50°C, separated into layers, removed raw materials, dehydrated the obtained solution under reduced pressure for 1-1.5 hours (about half of the original solution), cooled and crystallized to obtain 2-methoxy Benzoic acid 13.98g, yield 92%.
Embodiment 2
[0056] In a 500 mL three-necked flask, 7.57 g (0.2 mol) of sodium borohydride was dissolved in 283 mL of tetrahydrofuran solution, and stirred. Then, 15.2 g (0.1 mol) of 2-methoxybenzoic acid was added and reacted at 100° C. for 4 hours. Cool, add 100 mL of water, stir evenly and filter, the filtrate is recovered under reduced pressure, and precipitates are precipitated, and dried to obtain 13.13 g of white granular solid 2-methoxybenzyl alcohol with a yield of 95%. The reaction process is detected by TLC.
Embodiment 3
[0058] In a 250mL three-necked flask, sodium borohydride (19g, 0.5mol) was added into tetrahydrofuran (100mL, not purified) under ice-water bath conditions, followed by 30.4g (0.2mol) of 2-methoxybenzoic acid, and then added dropwise New H 2 SO 4 (13.2 mL, 0.5 mol) ether solution (40 mL in total). After the dropwise addition, stir at room temperature for 12 hours. Methanol (40 mL) was added dropwise, concentrated to half, followed by 20% NaOH (200 mL), and the solvent was spun off. The resulting mixture was refluxed for 3 hours. Cool, filter, and dilute to 400mL, extract 4 times with dichloromethane (150mL), wash with saturated sodium chloride (200mL), dry over anhydrous sodium sulfate, filter, distill out dichloromethane under reduced pressure, and distill the product with ethyl acetate (40 mL) / n-hexane (120 mL) recrystallized, filtered, and vacuum-dried to obtain 27.09 g of 2-methoxybenzyl alcohol as a white granular solid, with a yield of 98%. The reaction process was d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com