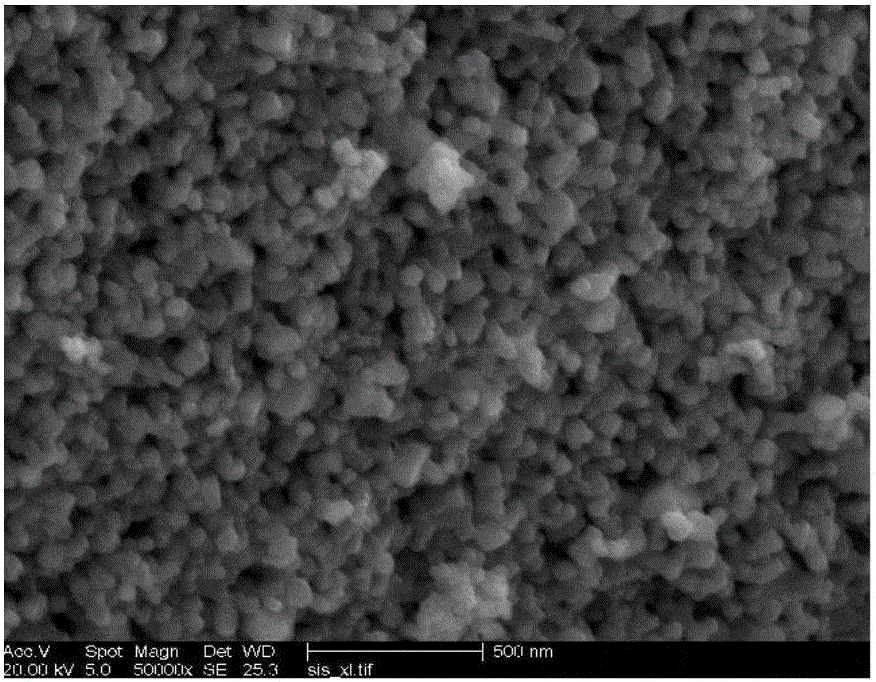
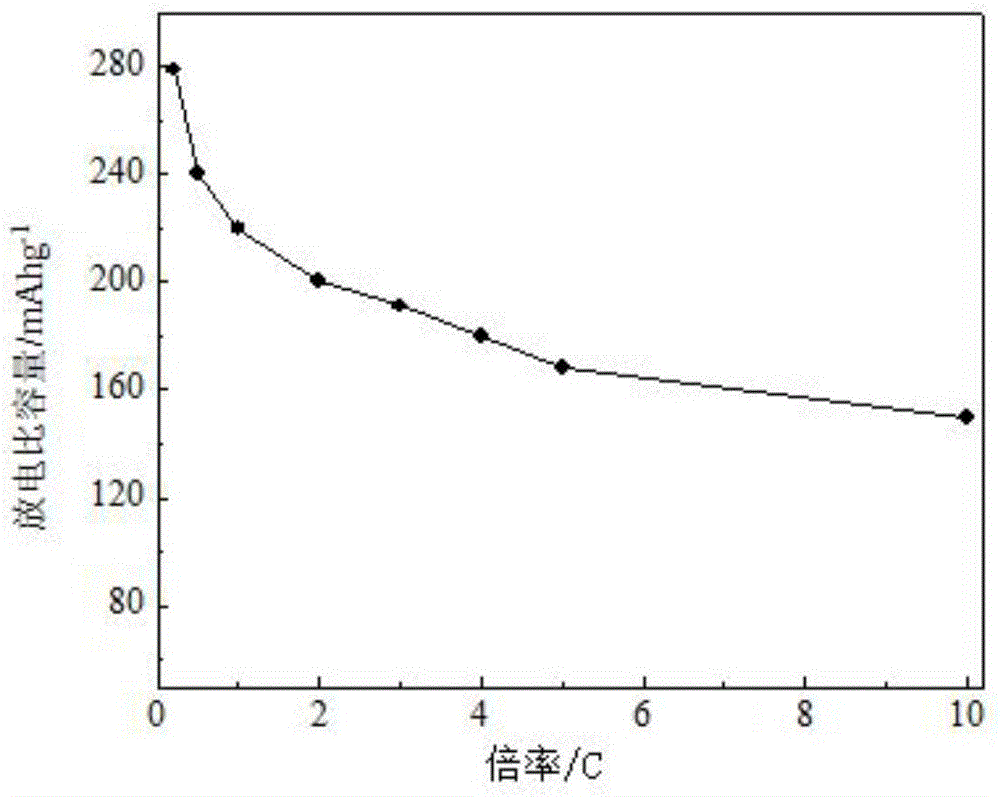
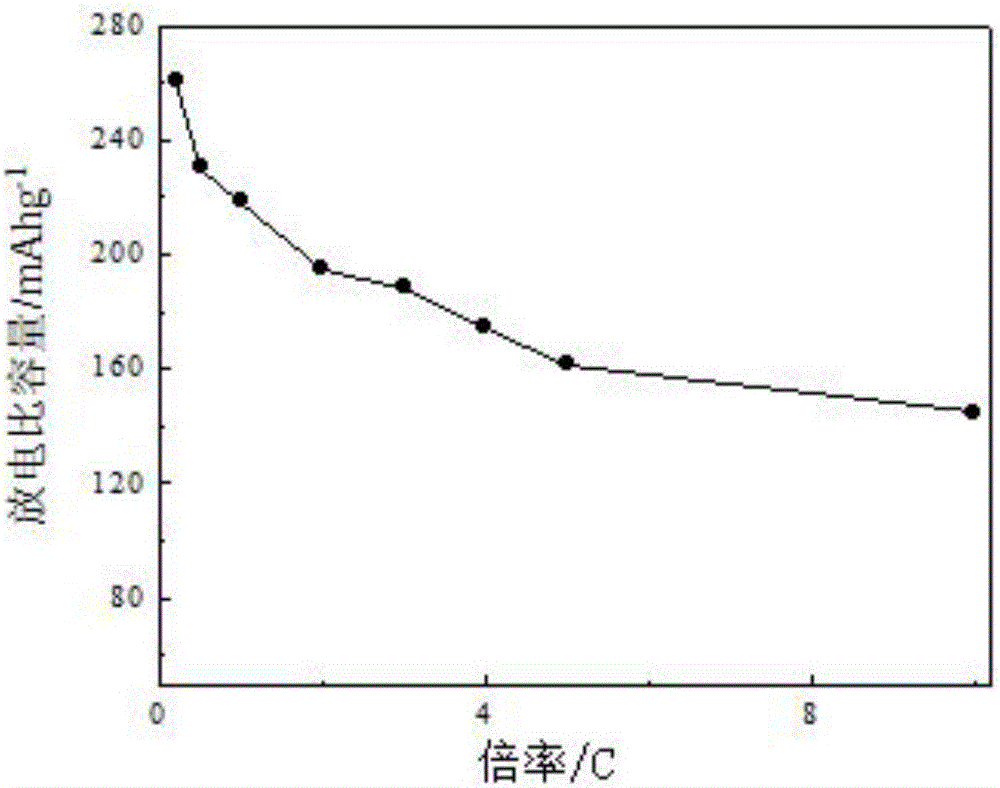
Lithium-rich anode material for lithium ion battery and preparation method thereof
A lithium-rich positive electrode material, lithium-ion battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of limiting the application of high-energy lithium-ion batteries, high irreversible capacity loss, and poor cycle stability, etc. Small irreversible capacity for the first time, avoiding uneven concentration distribution of the system, and good consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention provides a method for preparing a lithium-rich positive electrode material for a lithium ion battery described in the above technical solution, comprising the following steps:
[0042] Water-soluble cobalt salt, water-soluble nickel salt, water-soluble manganese salt, water-soluble cerium salt and water are mixed according to the molar ratio of 1-x-y-z-α:y:z:α to obtain the first solution, wherein 0<x <1, 0≤y<1, 0≤z<1, 0<α≤0.3;
[0043] mixing oxalic acid, urea and water to obtain a second solution;
[0044] Under the protection of an inert gas, the first solution is mixed with the second solution, and a co-precipitation reaction is performed to obtain an oxalate precursor;
[0045] After mixing the lithium source with the oxalate precursor, ball milling is carried out to obtain a mixed powder;
[0046] The mixed powder is calcined to obtain the lithium-rich positive electrode material of the lithium ion battery with the chemical composition show...
Embodiment 1
[0060] Weigh MnSO according to the ratio of Mn element, Ni element, Co element and Ce element molar ratio of 0.50:0.13:0.13:0.04 4 ·H 2 O, NiSO 4 ·6H 2 O. CoSO 4 ·7H 2 O and Ce(NO 3 ) 3 ·6H 2 O is dissolved in deionized water to obtain the first solution, and the total concentration of Mn element, Ni element, Co element and Ce element in the control solution is 1mol / L;
[0061] Mix diethyl oxalate and urea, control the molar concentration of diethyl oxalate to 1mol / L, and the molar ratio of urea to diethyl oxalate to 20:1 to obtain a second solution;
[0062] Under the protection of an inert gas, the first and second solutions were mixed respectively by a peristaltic pump, and the pH value was controlled within the range of 11.5±0.5 to carry out coprecipitation reaction, the reaction temperature was controlled at 90°C, stirred at a speed of 1000r / min for 12h, and filtered. Washing and drying in vacuum at 110°C for 12 hours to obtain the precipitation of the oxalate pre...
Embodiment 2
[0068] Weigh MnCl according to the ratio of Mn element, Ni element, Co element and Ce element molar ratio of 0.25:0.1:0.25:0.1 2 ·H 2 O, Ni(NO 3 ) 2 ·6H 2 O. CoSO 4 ·7H 2 O and Ce(NO 3 ) 3 ·6H 2 O is dissolved in deionized water to obtain the first solution, and the total concentration of Mn element, Ni element, Co element and Ce element in the control solution is 1mol / L;
[0069] Mix ammonium oxalate and urea, control the molar concentration of ammonium oxalate to 1.1 mol / L, and the molar ratio of urea to diethyl oxalate is 30:1 to obtain the second solution;
[0070]Under the protection of an inert gas, the first and second solutions were mixed separately by a peristaltic pump, and the pH value was controlled within the range of 10.5±0.5 for coprecipitation reaction. The reaction temperature was controlled at 70°C, stirred at a speed of 500r / min for 10h, and filtered. Washing and drying in vacuum at 110°C for 12 hours to obtain the precipitation of the oxalate precu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com