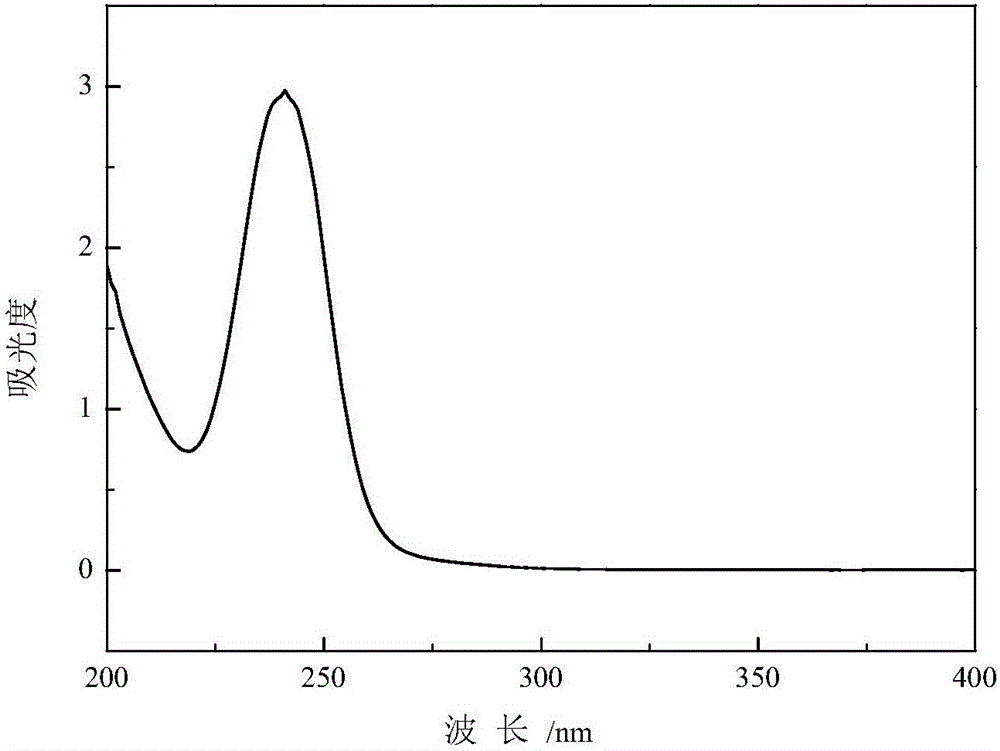
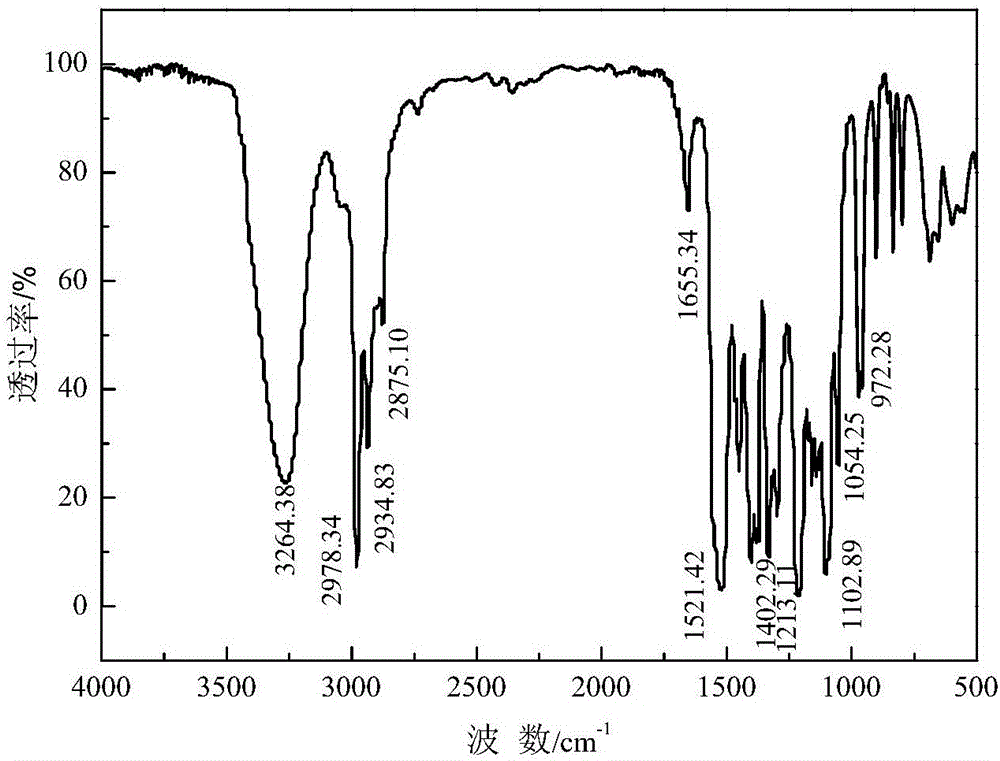
Method for preparing thionocarbamate and trithiocarbonate
A technology of trithiocarbonate and thiocarbamate, applied in the field of preparing thiocarbamate and co-producing trithiocarbonate, producing thiocarbamate and trithiocarbamate, which can solve the problem that has not yet been widely used , high cost of raw materials and other issues, to achieve the effect of high reactivity, increased yield, and easy precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Synthesis of O-isopropyl-N-ethylthiocarbamate and co-production of benzyl sodium trithiocarbonate:
[0061] 30.32 parts of purity are 95.12% of O-isopropyl-N-ethyl thiocarbamate and 17.53 parts of purity of 90.27% sodium isopropyl xanthate are added to the reactor, fully stirred, and then Add 12.79 parts of benzyl chloride with a purity of 99.0% into the pressure dropping funnel, control the reaction temperature to 75°C, and cool to room temperature after 3 hours of reaction, then add 6.68 parts of ethylamine aqueous solution (with a content of 65% to 70% %), warming up to 70 ° C, reacted for 2 hours, cooled to room temperature and filtered to remove sodium chloride, the filtrate was transferred to the reactor, and 8.08 parts of carbon disulfide with a purity of 99.0% were added to the reactor, and 4.38 parts of powdered hydroxide were added. Sodium (purity: 96.0%), after adding sodium hydroxide, stirred and reacted at 30° C. for 4 hours, and the reaction ended. Filter...
Embodiment 2
[0063] Synthesis of O-isobutyl-N-ethylthiocarbamate and co-production of benzyl sodium trithiocarbonate:
[0064] 25.30 parts of O-isobutyl-N-ethyl thiocarbamate with a purity of 94.77% and 18.84 parts of sodium isobutyl xanthate with a purity of 91.41% were added to the reactor, fully stirred, and then Add 12.79 parts of benzyl chloride with a purity of 99.0% to the pressure dropping funnel, control the reaction temperature to 65°C, react for 2 hours, cool to room temperature, and then add 6.68 parts of ethylamine aqueous solution (with a content of 65% to 70% %), warming up to 70 ° C, reacted for 2 hours, cooled to room temperature and filtered to remove sodium chloride, the filtrate was transferred to the reactor, and 8.08 parts of carbon disulfide with a purity of 99.0% were added to the reactor, and 4.38 parts of powdered hydroxide were added. Sodium (purity: 96%), after adding the caustic, stirred and reacted at 30° C. for 4 hours, and the reaction ended. Filter to obta...
Embodiment 3
[0066] Synthesis of O-isopropyl-N-propylthiocarbamate and co-production of benzyl sodium trithiocarbonate:
[0067] 114.95 parts of O-isopropyl-N-propyl thiocarbamate with a purity of 95.12% and 53.59 parts of sodium isopropyl xanthate with a purity of 90.27% were added to the reactor, fully stirred, and then Add 38.37 parts of purity to 99.0% benzyl chloride into the pressure dropping funnel, control the reaction temperature to be 75°C, react for 3 hours, cool to room temperature, then add 18.09 parts of 98.0% n-propylamine with a constant pressure dropping funnel, and heat up to 70 ℃, reacted 3 hours, cooled to room temperature and filtered to remove sodium chloride, the filtrate was transferred to the reactor, added 25.38 parts of carbon disulfide with a purity of 99.0% in the reactor, and added 13.13 parts of powdered sodium hydroxide (purity is 96 %), after adding the caustic alkali, stirred and reacted at 30°C for 4 hours, and the reaction ended. Filter to obtain benzyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com