Double-effect organic macromolecular compound and preparation method thereof
A technology of organic macromolecules and compounds, applied in the field of fine chemicals, can solve the problems of cumbersome post-treatment process and high cost, and achieve the effects of improving net capture ability, inhibiting corrosion and fast flocculation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
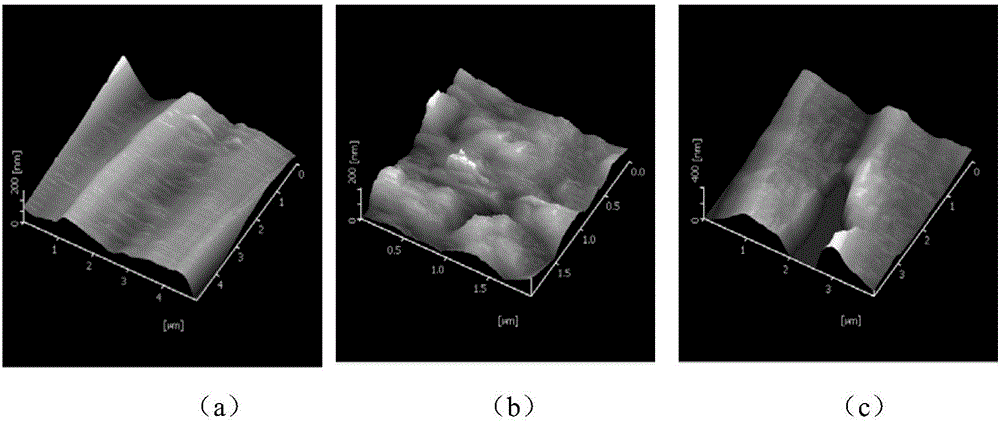
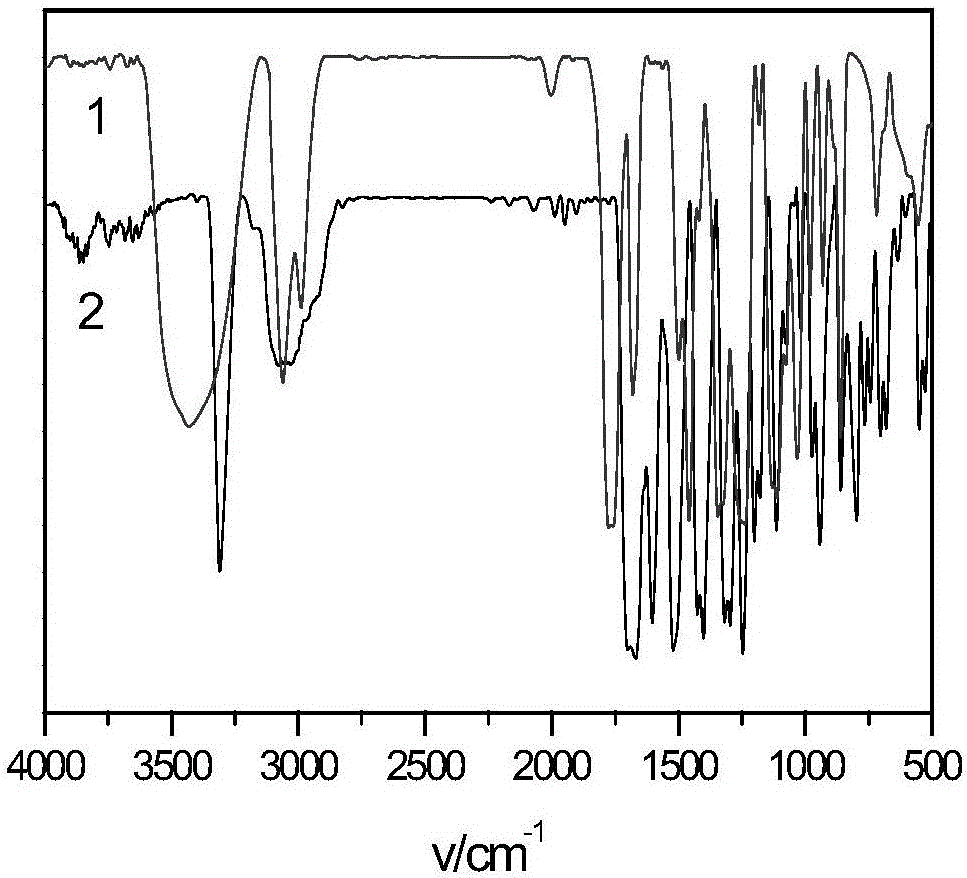
Image
Examples
Embodiment 1
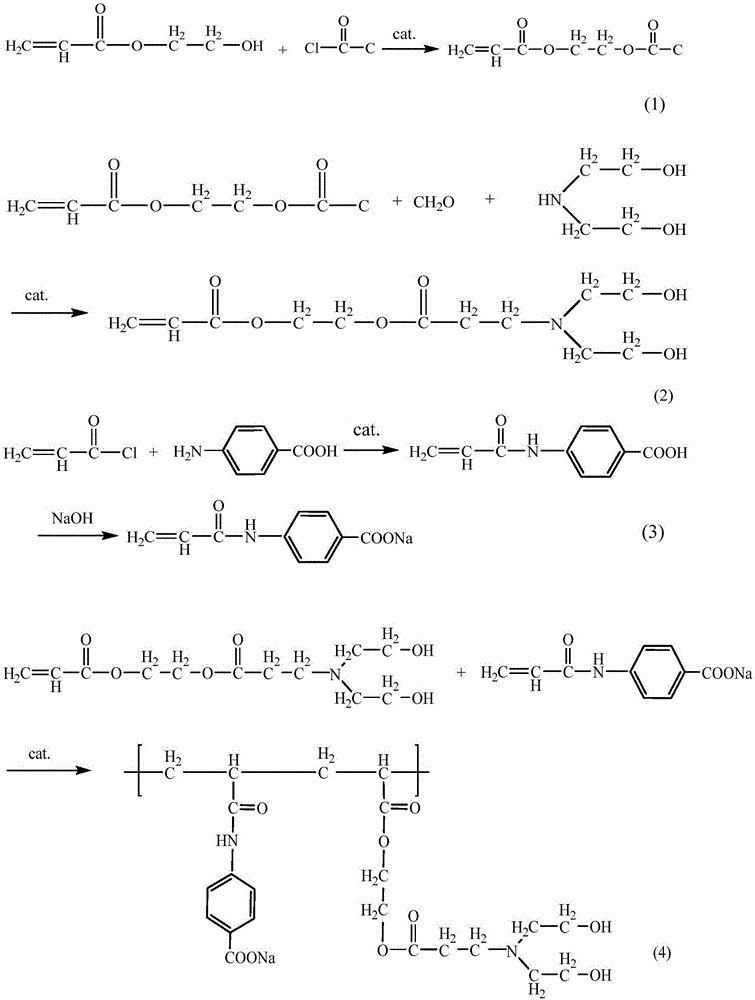
[0040] The preparation of double-effect organic macromolecular compound comprises the following steps:
[0041] 1. Sodium p-acrylamide benzoate is obtained by the following method:
[0042] Add 13.7g of p-aminobenzoic acid and 30mL of ethyl acetate into a three-necked flask equipped with a stirrer, a constant pressure dropping funnel and a condenser, and slowly add 13.5g of acryloyl chloride dropwise with a constant pressure funnel at 0°C. And fully stirred, after reacting for 2 hours, the solvent ethyl acetate was removed by suction filtration, and washed twice with ethyl acetate to obtain p-acrylamide benzoic acid, which was then slowly neutralized to p-acrylamide benzoic acid with a mass fraction of 35% sodium hydroxide solution. The formic acid is completely dissolved, the water is distilled off, dried and pulverized to obtain sodium p-acrylamide benzoate;
[0043] Described Mannich base is made by following method:
[0044] (a) After mixing 11.16g of hydroxyethyl acryla...
Embodiment 2
[0049] The preparation of double-effect organic macromolecular compound comprises the following steps:
[0050] 1. Sodium p-acrylamide benzoate is obtained by the following method:
[0051] Add 13.7g of p-aminobenzoic acid and 20mL of ethyl acetate into a three-neck flask equipped with a stirrer, a constant pressure dropping funnel and a condenser tube, and slowly add 9g of acryloyl chloride dropwise with a constant pressure funnel at 10°C, and Stir well, and react for 1 hour, remove the solvent ethyl acetate by suction filtration, and wash twice with ethyl acetate to obtain p-acrylamide benzoic acid, then slowly neutralize to p-acrylamide benzoic acid with 45% sodium hydroxide solution Completely dissolved, distilled to remove water, dried and pulverized to obtain, sodium p-acrylamide benzoate;
[0052] Described Mannich base is made by following method:
[0053] (a) After mixing 11.6 g of hydroxyethyl acrylate and 6.06 g of triethylamine, place it in an ice-water bath, sti...
Embodiment 3
[0058] The preparation of double-effect organic macromolecular compound comprises the following steps:
[0059] 1. Sodium p-acrylamide benzoate is obtained by the following method:
[0060] Add 13.7g of p-aminobenzoic acid and 25mL of ethyl acetate into a three-necked flask equipped with a stirrer, a constant pressure dropping funnel and a condenser, and slowly add 10.8g of acryloyl chloride dropwise with a constant pressure funnel at 20°C. And fully stirred, after reacting for 1.5h, the solvent ethyl acetate was removed by suction filtration, and washed twice with ethyl acetate to obtain p-acrylamide benzoic acid, and then slowly neutralized to p-acrylamide with 50% sodium hydroxide solution The benzoic acid is completely dissolved, and the water is distilled off, dried and pulverized to obtain sodium p-acrylamide benzoate;
[0061] Described Mannich base is made by following method:
[0062] (a) After mixing 11.6 hydroxyethyl acrylate with 5g sodium hydroxide, place it in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com