Application of adopting aspirin conjugate with antitumor activity as drug carrier or molecular probe carrier
A technology of aspirin and molecular probes, which is applied in the application field of aspirin conjugates as drug carriers or molecular probe carriers, can solve problems such as difficult quality control, complex nanosystems, and unclear mechanism of action, and achieve simple and convenient synthesis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Synthesis of aspirin-betulinic acid
[0062] At room temperature, 0.5 g of acetylsalicylic acid (aspirin) was dissolved in 20 mL of dry dichloromethane, 2 mL of oxalyl chloride was added, magnetically stirred for 10 h, and the gas and solvent were evaporated under reduced pressure. Add 20 mL of dichloromethane to the reaction flask again, and add dropwise to 20 mL of 1v / 1v pyridine:dichloromethane mixed solution dissolved with 1.27 g of betulinic acid and 0.1eq DMAP through a constant pressure funnel, and continue to stir after the dropwise addition is completed 8-10 h. After the reaction was complete, dichloromethane was distilled off under reduced pressure. Add 100 mL of water to the reaction flask to precipitate the product, filter it with suction, wash the filter cake with 500 mL of water until neutral, dry it in vacuum, and obtain aspirin-betulinic acid (Asp-BA) by column chromatography.
[0063] Properties: white powder; yield: 70.51%;
[0064] Molecular formul...
Embodiment 2
[0068] Preparation method of Asp-BA nanoparticles
[0069] Accurately weigh 0.00618g of Asp-BA powder, dissolve it in 1 ml of methanol, and dissolve it by ultrasonic to make a 10 mM solution; take 20 µL of methanol solution and add it dropwise to the EP tube containing 180 µL (Note: drop Vortex during the addition process, 20 µL dropwise addition time is 20s), then. After ultrasonication for 1 min, centrifugation at 12000 rpm for 5 min, the supernatant was Asp-BA nanoparticles.
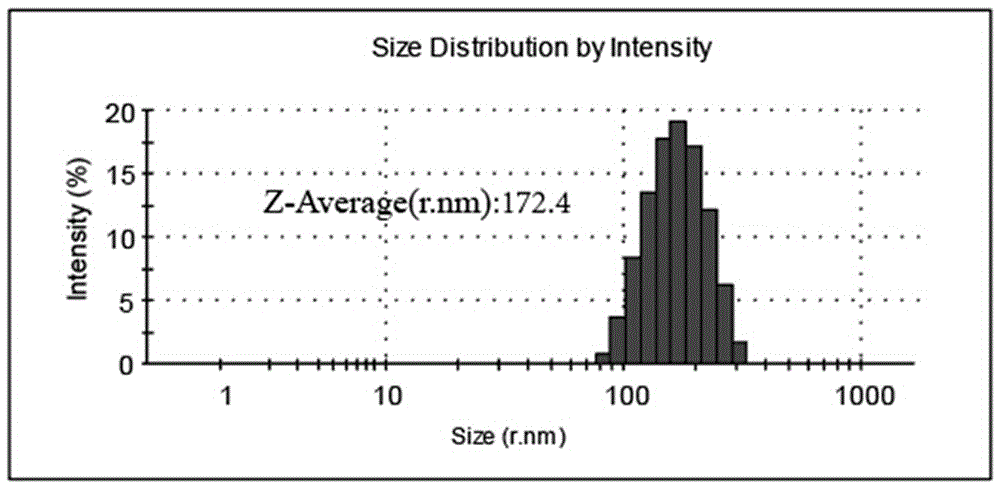
[0070] The average size of the particle diameter of the Asp-BA nanoparticle aqueous solution prepared in this embodiment is about 170 nanometers, and the particle diameter diagram is as follows figure 1 shown.
[0071] Because most of the currently approved anticancer nanomedicine particles are in the size range of 100-200 nm, and within a certain range, smaller sized anticancer nanomedicine particles show stronger anticancer properties in vivo.
Embodiment 3
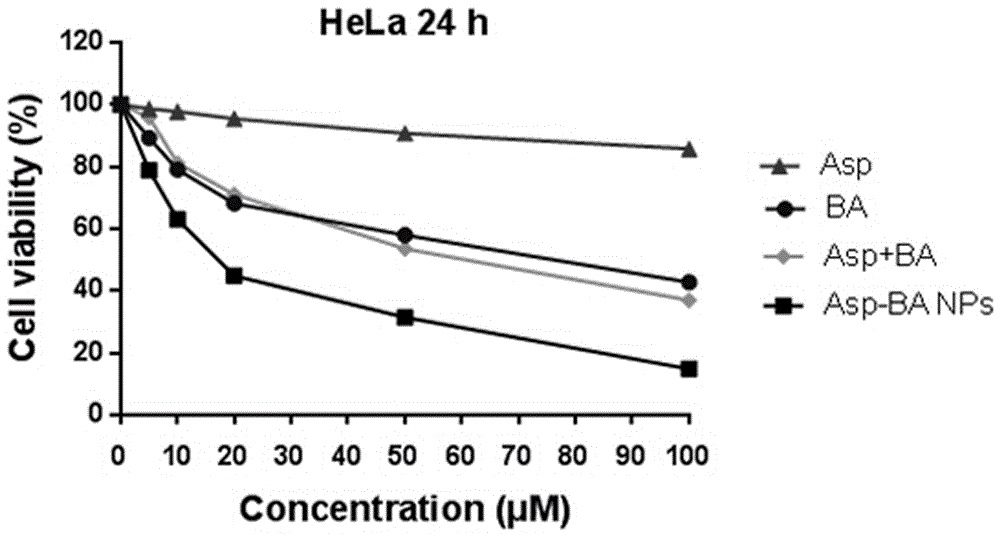
[0073] The anticancer activity experiment was realized by cytotoxicity, and the proliferation inhibitory activity of Asp-BA nanoparticles, Asp, BA solution and composition on HeLa cells was measured by standard MTT colorimetric method. The specific steps are: (1) Take HeLa cells in good logarithmic growth phase, digest with trypsin, count and adjust the cell density to 0.8×105 cells / mL, and make a cell suspension. Inoculate 100 µL per well into a 96-well plate, seal the plate with PBS, and store at 37 °C, 5% CO 2 Cultivate in the incubator for 24h.
[0074] (2) Remove the old culture medium, add 100 µL of culture medium containing samples with different concentration gradients to each well, set up a blank control group, set 5 replicate wells in each group, and continue to incubate in the incubator for 24 hours.
[0075] (3) Remove the medium, add 100 µL of MTT solution (serum-free, phenol red-free RMPI1640 medium: MTT mother solution = 9:1, V:V) to each well, and continue to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com