Thiophene compound, preparation method and application thereof and perovskite solar battery
A technology of solar cells and compounds, applied in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of low hole mobility, high price, unstable battery device performance, etc., and achieve high hole mobility and high energy conversion. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention also provides a method for preparing the above-mentioned thiophene compounds, comprising:
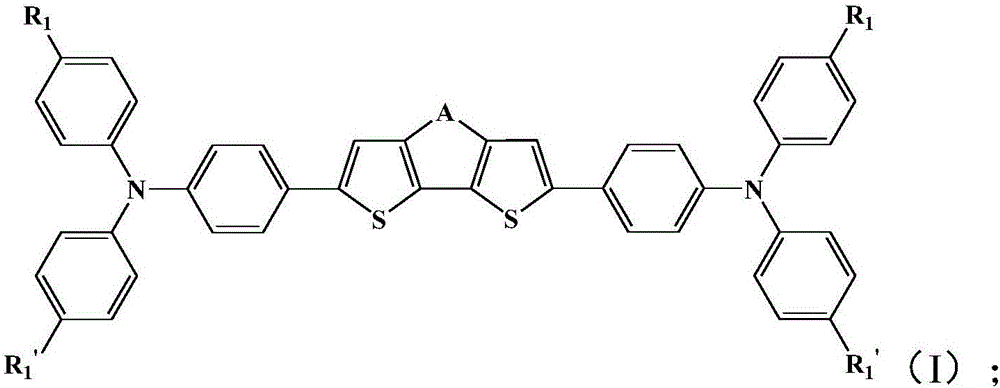
[0049] The compound shown in formula (II) is reacted with the compound shown in formula (III) to obtain the thiophene compound shown in formula (I);
[0050]
[0051]
[0052] Where -A- is -S-,
[0053] X is a halogen atom, preferably Cl or Br, more preferably Br;
[0054] R 1 Is H, C1~C6 hydrocarbon group or C1~C6 alkoxy group;
[0055] R 2 , R 3 with R 4Each is independently H, a C1-C6 hydrocarbon group, a C1-C6 alkoxy group or a phenyl group substituted by a C1-C6 alkoxy group.
[0056] The R 1 , R 2 , R 3 with R 4 All are the same as above, and will not be repeated here.
[0057] According to the present invention, the conditions of the reaction are Suzuki coupling reaction conditions well known to those skilled in the art, and there is no special limitation.
[0058] The organic hole transport material based on the fused ring thiop...
Embodiment 1
[0073]
[0074] In a dry Schlenk reaction flask, add 1 g of intermediate 1, 4.87 g of 4-methoxy-N-(4-methoxyphenyl)-N-(4-(4,4,5,5-tetra Methyl-1,3,2-dioxaborolan-2-yl)phenyl)amine, 3.59g sodium carbonate, then add 50mL toluene, 50mL water to dissolve, add 130mg Pd(PPh 3 ) 4 , heated to reflux, and reacted overnight under magnetic stirring.
[0075] After the reaction was detected by TLC, the reaction system was cooled to room temperature, water was added, and extracted three times with ethyl acetate, the organic phase was combined, and the organic phase was dried with anhydrous sodium sulfate, the desiccant was filtered off, spin-dried, and then washed with toluene / petroleum ether ( Volume ratio 4:1) was used as a developer for column chromatography to obtain 1.5 g of a yellow solid powder target product, ie, a thiophene compound represented by formula (I-1).
[0076] The structure of the obtained target product formula I-1 was characterized by nuclear magnetic resonance,...
Embodiment 2
[0081]
[0082] 2.1 Synthesis of the thiophene compounds shown in the target product formula (I-2)
[0083] In a dry Schlenk reaction flask, 720 mg of intermediate 2 was added and dissolved in 10 mL of tetrahydrofuran. After the ice bath was complete, 1.12 g of nitrogen bromosuccinimide was added, and the mixture was reacted for 30 min under magnetic stirring, and the reaction was detected by TLC. Add 20 mL of water, extract the reaction mixture with chloroform, combine the organic phases, dry the organic phases with anhydrous sodium sulfate, filter off the desiccant, and spin dry the solvent. The white solid powder obtained is directly used in the next reaction.
[0084] In a dry Schlenk reaction flask, add 600 mg of the product obtained in the above reaction, and then add 1.614 g of 4-methyl-N-(4-methylphenyl)-N-(4-(4,4,5,5-tetra Methyl-1,3,2-dioxaborolan-2-yl)phenyl)amine, 1.43g potassium phosphate, then add 20mL dioxane, 4mL water to dissolve, add 7.26mg Pd( OAC) 2 13...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com