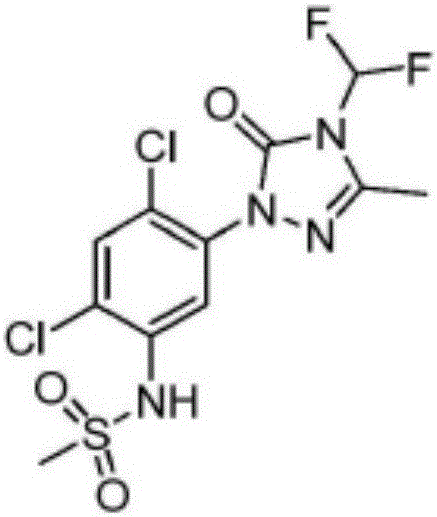
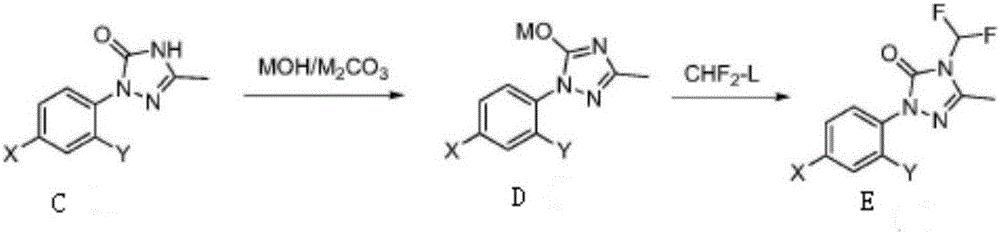
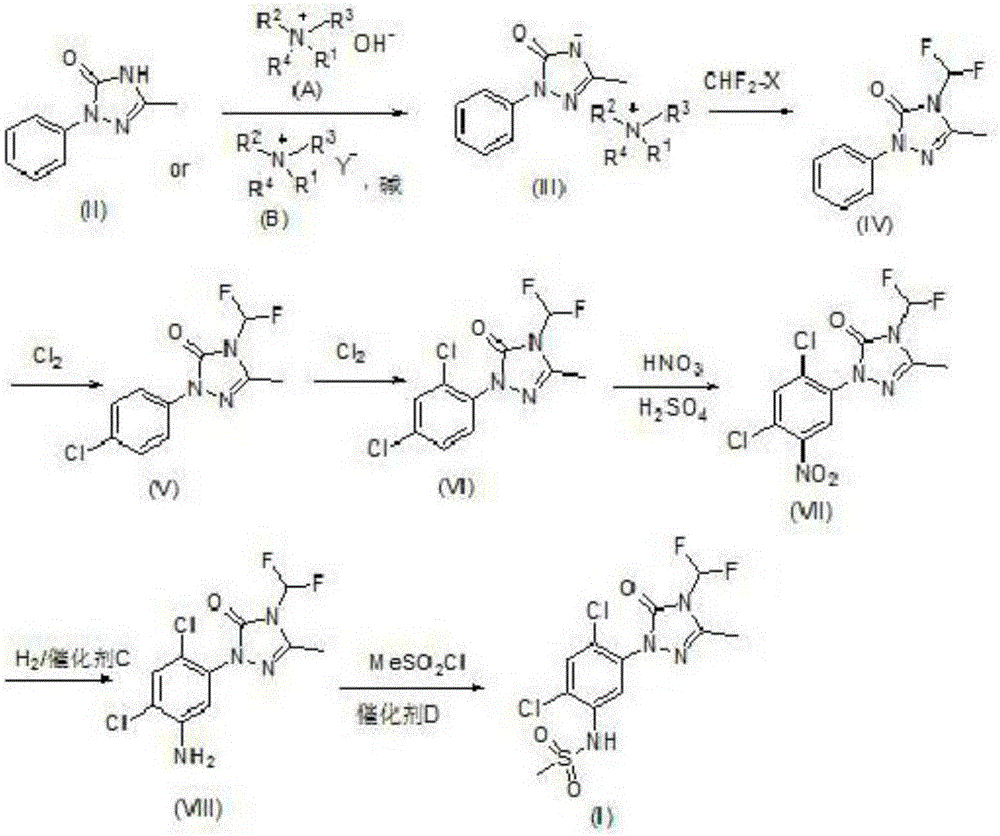
Method for synthesizing Sulfentrazone
A technology of sulfentranacil and methyl, which is applied in the field of synthesizing sulfentranacil, can solve the problems of easy occurrence of steric hindrance in intermediate products, recycling of unfavorable solvents, poor positioning effect, etc., and achieve good positioning effect, small steric hindrance, Benefits of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of 1-o-chlorophenyl-3-methyl-1H-1,2,4-triazol-5-one:
[0035] Dissolve 15g of o-chlorophenylhydrazine hydrochloride in 240g of a dilute solution of 5% potassium carbonate, and extract 2 to 3 times with 500mL of dichloromethane, and add 11g of trimethyl orthoacetate and 100mL of methanol to the extracted organic matter , Stir and reflux for 1-2 hours, then cool to 20-30°C, then add 8g of potassium cyanate and stir for 30-40min, when the temperature drops to 0-2°C, add 6g of glacial acetic acid dropwise, and under the condition of 20-30°C Stirring at low temperature for 18-20 hours, 9g of 1-o-chlorophenyl-3-methyl-1H-1,2,4-triazol-5-one was obtained, calculated as o-chlorophenylhydrazine hydrochloride, 1-o-chlorophenyl The yield of base-3-methyl-1H-1,2,4-triazol-5-one is 51.5%; its specific reaction is as follows:
[0036]
[0037]
Embodiment 2
[0039] Synthesis of 1-o-chlorophenyl-3-methyl-4-difluoromethyl-1,2,4-triazol-5-one:
[0040] Put 16g of 1-o-chlorophenyl-3-methyl-1H-1,2,4-triazol-5-one into the reaction bottle A, and then add 7.3g of potassium hydroxide powder, 10.5g of tetrabutyl bromide Amine and 150mL tetrahydrofuran, stirred and reacted, and when the temperature was raised to 75-85°C, 10g of difluorochloromethane was introduced. After the reaction, cooled to room temperature, tetrahydrofuran was evaporated, then added with 60mL of water, filtered, and dried to obtain 18g of 1-o-chlorobenzene Base-3-methyl-4-difluoromethyl-1,2,4-triazol-5-one, with 1-o-chlorophenyl-3-methyl-1H-1,2,4-triazole Based on -5-ketone, the yield of 1-o-chlorophenyl-3-methyl-4-difluoromethyl-1,2,4-triazol-5-one is 90.7%; its specific reaction is as follows:
[0041]
Embodiment 3
[0043] Synthesis of 1-(2,4-dichlorophenyl)-3-methyl-4-difluoromethyl-1,2,4-triazol-5-one:
[0044] Add 30g of 1-o-chlorophenyl-3-methyl-4-difluoromethyl-1,2,4-triazol-5-one, 150g of dichloroethane and 0.2g of composite catalyst trichloro in reaction flask B Antimony, ferric chloride and silicon dioxide, and lower the temperature to 8 ~ 10 ° C, and then react with 13g of chlorine, after the reaction, add 100mL of water to obtain 33g of 1-(2,4-dichlorophenyl)-3- Methyl-4-difluoromethyl-1,2,4-triazol-5-one, with 1-o-chlorophenyl-3-methyl-4-difluoromethyl-1,2,4-tri Based on oxazol-5-one, the yield of 1-(2,4-dichlorophenyl)-3-methyl-4-difluoromethyl-1,2,4-triazol-5-one is 97.1% ; Its specific reaction is as follows:
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com