High-throughput enzyme sensor and method for detecting urea in human urine
The technology of an enzyme sensor and detection method is applied in the direction of material analysis by observing the influence on chemical indicators, and analysis by causing the material to undergo chemical reaction. and other problems, to achieve the effect of repeated use, qualitative and quantitative detection, and reduction of experimental costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Sources of reagents and equipment used:
[0023] 1 Robolids microplate and 96 microplate: Sigma-Aldrich;
[0024] 2 Urease, chitosan: Sigma Aldrich (Shanghai);
[0025] 3 Sodium alginate: Tianjin Guangfu Fine Chemical Research Institute;
[0026] 4 Nessler's reagent, urea: Aladdin;
[0027] 4 High-throughput pipette: Decong Scientific Instrument Co., Ltd.;
[0028] 5 Epoch2 Microplate Reader: Burton, USA
[0029] 6 K5600 miniature ultraviolet-visible spectrophotometer: Beijing Kaiao Technology Development Co., Ltd.
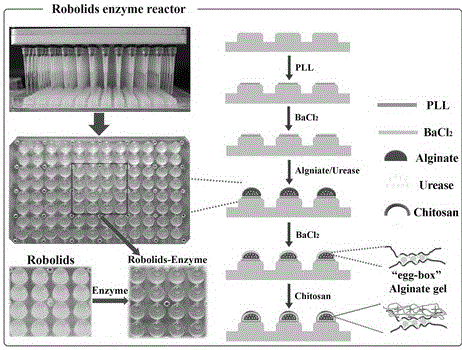
[0030] Preparation of a novel three-dimensional high-throughput enzyme reactor:
[0031] 1) Robolids plate pretreatment
[0032] Seal the commercial Robolid sealing plate (96 silica gel microcolumns, diameter 5mm / column, height 5mm / column) in a 96-microliter microcolumn containing 50uL 0.01% (w / v) poly-lanine (PLL) solution in each well. On the orifice plate, invert the surface of each microcolumn of Robolid to be covered with PLL solution, and inc...
Embodiment 2
[0038] Construction of Urea Standard Curve and Monitoring of Urease Kinetics
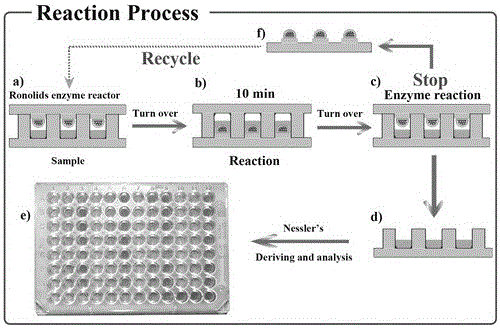
[0039] 1) Use 50 mM phosphate buffer solution (pH 7.0) to prepare a series of urea standard substances with different concentrations, add them to the microwell plate one by one, seal the microwell plate with the prepared urease immobilized robolid plate, and invert it to make the sample to be tested contact with the enzyme Start the enzyme reaction at 37°C for 10 min, then invert to separate the sample to be tested from the enzyme to stop the enzyme reaction.
[0040] 2) 24 uL of Nessler's reagent was distributed to the enzyme wells of the microplate in 1) above, and the color reaction was carried out at room temperature for 10 min.
[0041] 3) Use an Epoch2 microplate reader to detect the chromogenic product in 2), and output the absorbance value at a wavelength of 405 nm.
[0042] 4) Draw the standard curve with the absorbance at 405 nm wavelength as the ordinate and the urea concentration as the...
Embodiment 3
[0045] Actual sample testing
[0046] A urine sample was diluted 100 times, and urea standard samples of different known concentrations were added, and the recovery rate of the calculated standard addition was between 95.3% and 104%, as shown in Table 2.
[0047]28 actual urine samples with unknown concentrations were diluted 100 times, 150 times and 200 times respectively for detection with the high-throughput enzyme sensor prepared by the present invention. Substitute the absorbance into the standard curve, and compare the obtained result with the result obtained by the standard urease method, such as Figure 8 It shows that the method has high accuracy.
[0048] Table 1: Comparison of the method of the present invention with other urea detection methods
[0049]
[0050] Table 2: Recovery of standard spiked urea in human urine and ultrapure water
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com