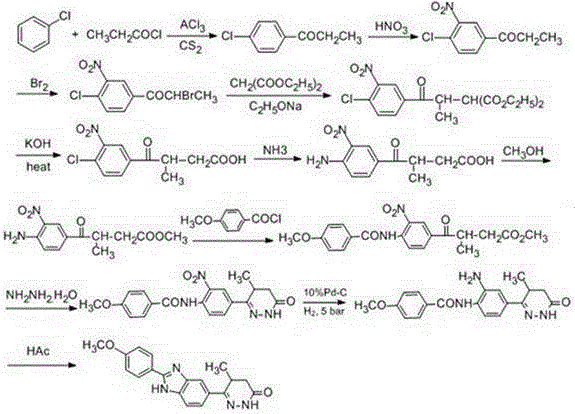
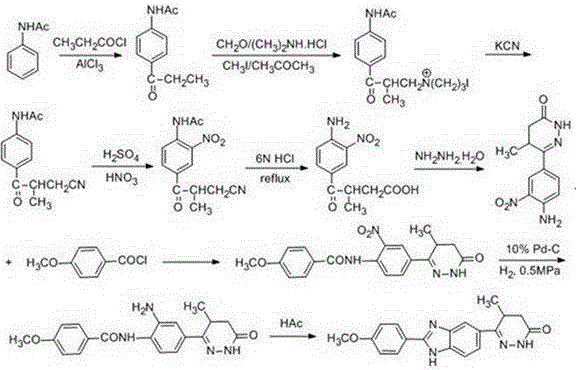
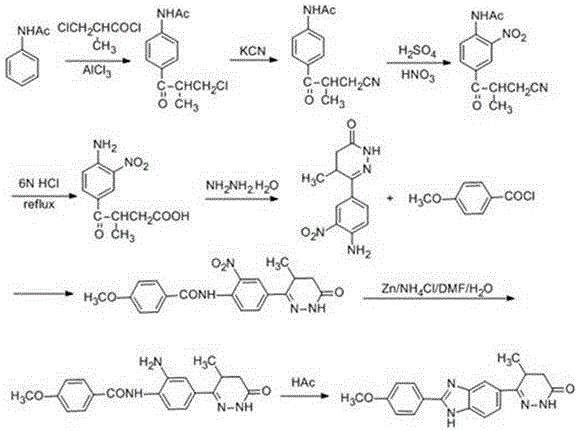
Chemical synthesis method of Pimobendan
A technology for chemical synthesis and pimobendan, applied in the direction of organic chemistry and the like, can solve the problems of unsuitable large-scale preparation of pimobendan, long reaction route, potential safety hazards, etc., and achieves low cost, short reaction period, corrosiveness and the like. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036]Step ①: Dissolve acetanilide (0.2 mol, 27g) in a mixed solvent of 45mL carbon disulfide and 45mL dichloromethane, stir at room temperature to obtain a colorless transparent solution, and add anhydrous aluminum trichloride (0.4 mol, 52.4g ) and potassium iodide (0.01moL, 1.65g), heated to reflux for 1h, cooled to room temperature, added 2-methyl-3-methoxycarbonylpropionyl chloride (0.21moL, 34.4g), reacted at 70°C for 8h, concentrated under reduced pressure and recovered Solvent, the residue was poured into 800mL ice water containing 13ml of concentrated hydrochloric acid, left at room temperature for 2h, a light yellow precipitate was precipitated, filtered, and the precipitate was collected to obtain the crude product of 3-acetamidobenzoyl butyric acid methyl ester, which was weighed with 95% ethanol Crystallization gave 43.1 g of methyl 3-p-acetamidobenzoyl butyrate, yield 82%, melting point 212-213°C; 1HNMR (500MHz, DMSO-d6) δ1.23 (d, J=6.8Hz, 3H, CH3 ), 2...
Embodiment 2
[0048] Step ①: Dissolve acetanilide (0.2 mol, 27g) in a mixed solvent of 45ml carbon disulfide and 45ml N,N-dimethylformamide, stir at room temperature to obtain a colorless transparent solution, and add anhydrous trichloro Aluminum chloride (0.4 mol, 52.4g) and potassium iodide (0.015 mol, 2.48g), heated to reflux for 1h, cooled to room temperature, added 2-methyl-3-methoxycarbonyl propionyl chloride (0.21 mol, 34.4g), at 70 React at ℃ for 8 hours, concentrate under reduced pressure to recover the solvent, pour the residue into 800mL ice water containing 13ml of concentrated hydrochloric acid, leave it at room temperature for 2h, a light yellow precipitate precipitates, filter, collect the precipitate to obtain 3-acetamidobenzoyl butyric acid methyl ester The crude product was recrystallized from 95% ethanol to obtain 46.3 g of methyl 3-p-acetamidobenzoyl butyrate, with a yield of 88% and a melting point of 212-213°C.
[0049] Step ②: Mix the 3-p-acetamidobenzoyl butyric acid...
Embodiment 3
[0055] Step ①: Dissolve acetanilide (0.2 mol, 27g) in a mixed solvent of 45ml carbon disulfide and 45ml N,N-dimethylformamide, stir at room temperature to obtain a colorless transparent solution, and add anhydrous trichloro Aluminum chloride (0.4 mol, 52.4g) and potassium iodide (0.02 mol, 3.3g), heated to reflux for 1h, cooled to room temperature, added 2-methyl-3-methoxycarbonyl propionyl chloride (0.21 mol, 34.4g), at 70 React at ℃ for 8 hours, concentrate under reduced pressure to recover the solvent, pour the residue into 800mL ice water containing 13ml of concentrated hydrochloric acid, leave it at room temperature for 2h, a light yellow precipitate precipitates, filter, collect the precipitate to obtain 3-acetamidobenzoyl butyric acid methyl ester The crude product was recrystallized from 95% ethanol to obtain 46.3 g of methyl 3-p-acetamidobenzoyl butyrate, with a yield of 88% and a melting point of 212-213°C.
[0056] Step ②: Mix the 3-p-acetamidobenzoyl butyric acid m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com