A kind of measuring method of ammonia nitrogen concentration value in water body
A technology of ammonia nitrogen concentration and determination method, which can be applied to measurement devices, preparation of test samples, and color/spectral characteristics measurement, etc., can solve problems such as adverse human health, reduced oxygen transport, tissue damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
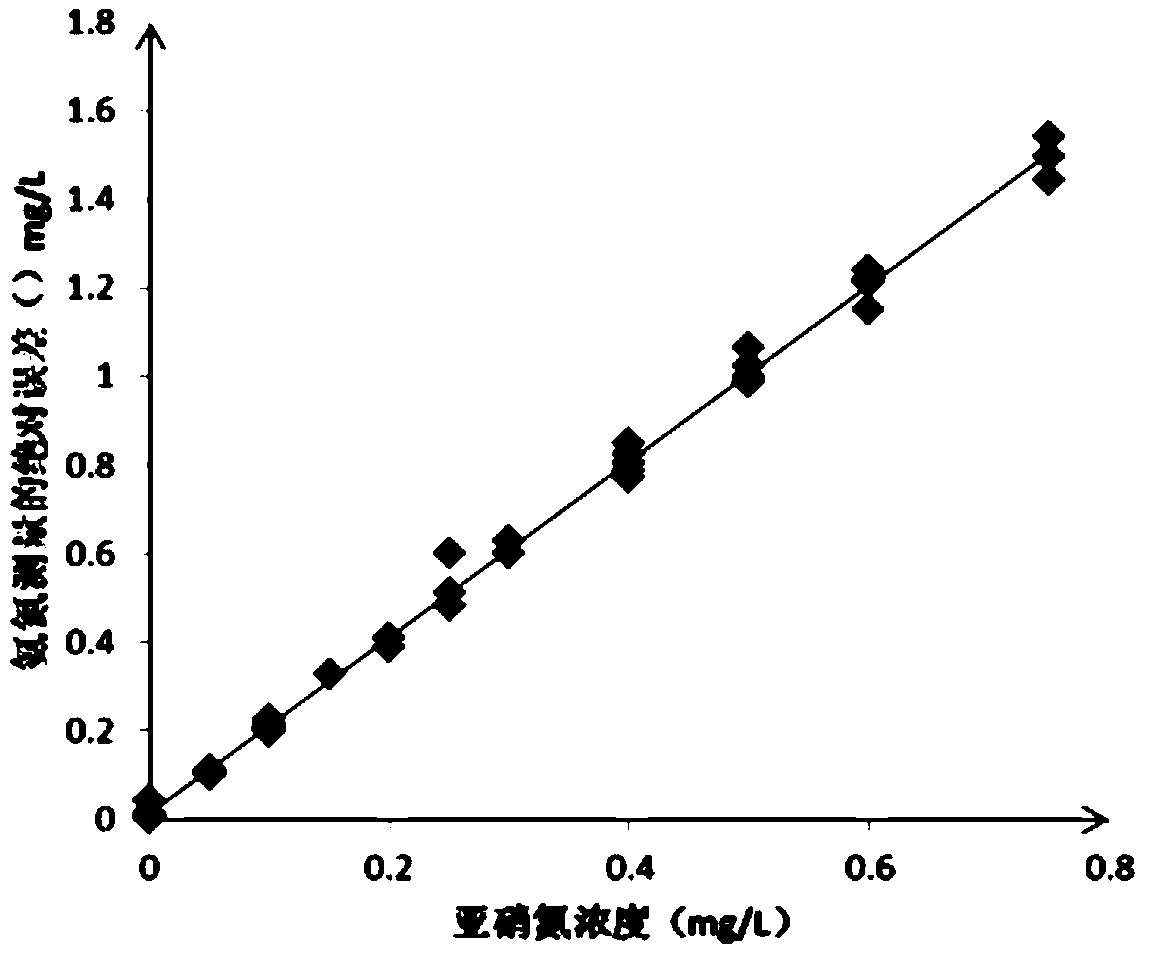
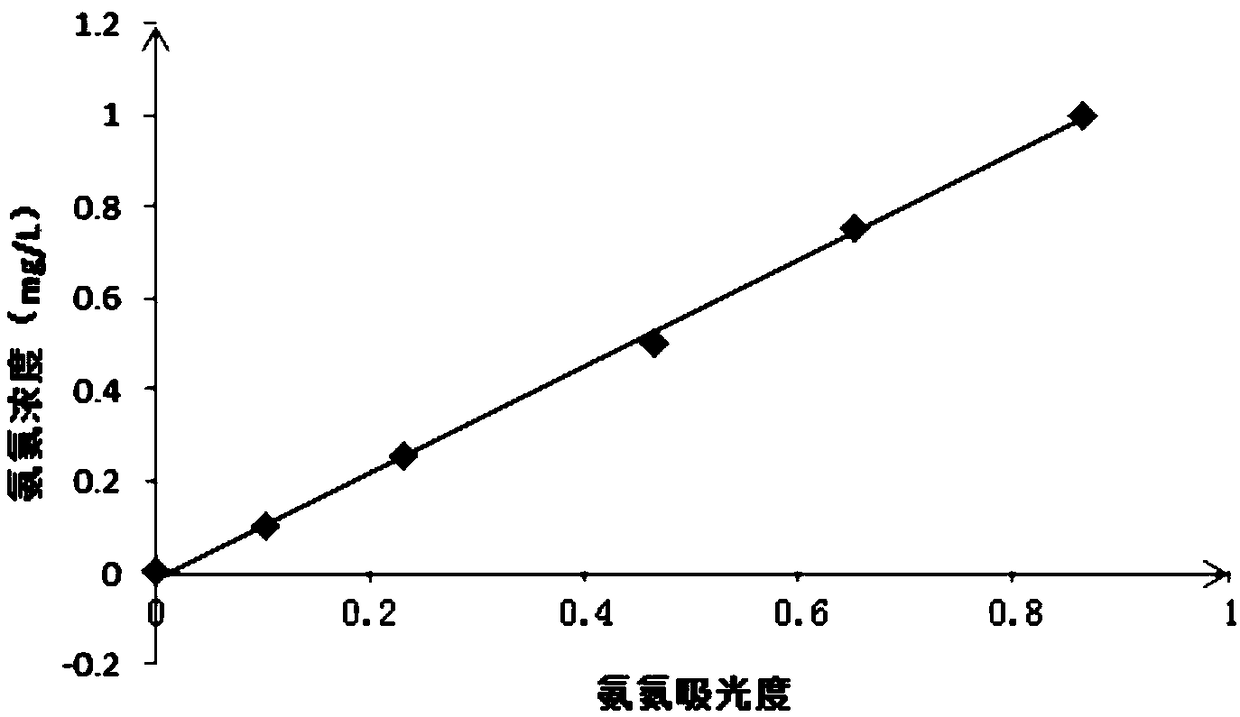
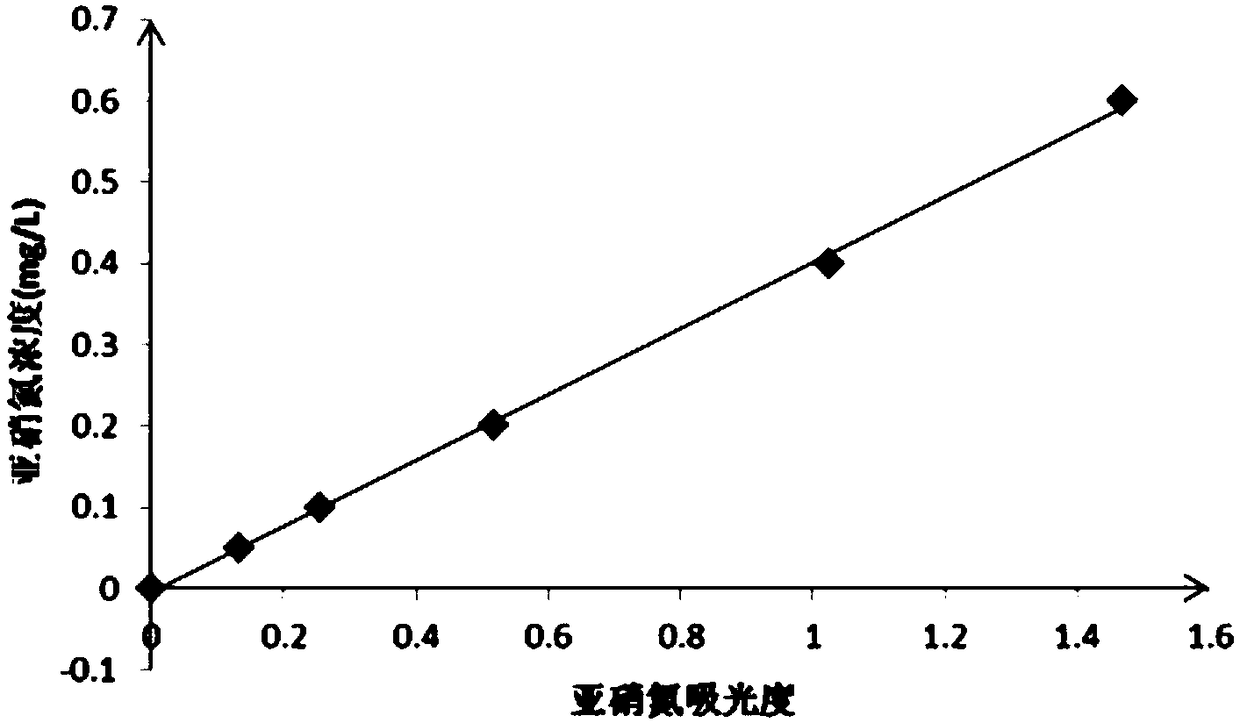
[0034] The process engineering of the method for measuring the concentration of ammonia nitrogen in water involved in this embodiment includes five steps: preparatory work, drawing a standard curve of ammonia nitrogen, drawing a standard curve of nitrous nitrogen, drawing a curve of the influence of nitrite nitrogen concentration on the absolute error of ammonia nitrogen measurement, and measuring the concentration of ammonia nitrogen :
[0035] (1), the preparatory work includes preparation of hydrochloric acid solution, preparation of p-aminobenzenesulfonamide hydrochloric acid solution, preparation of naphthaleneethylenediamine hydrochloride aqueous solution, preparation of nitrous nitrogen color reagent, preparation of ammonia nitrogen standard solution, preparation of nitrous nitrogen standard solution and preparation of secondary Sodium bromate solution:
[0036] 1. Preparation of hydrochloric acid solution: Dissolve 100mL of concentrated hydrochloric acid with a mass pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com