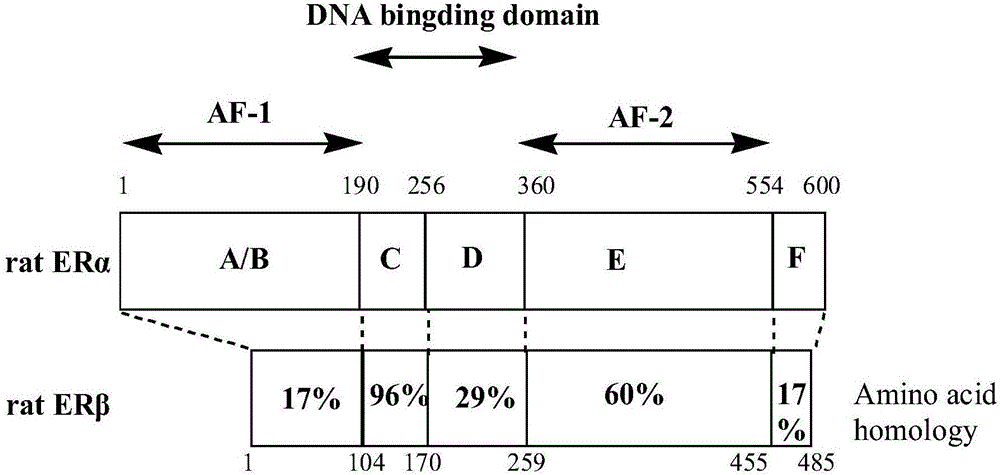
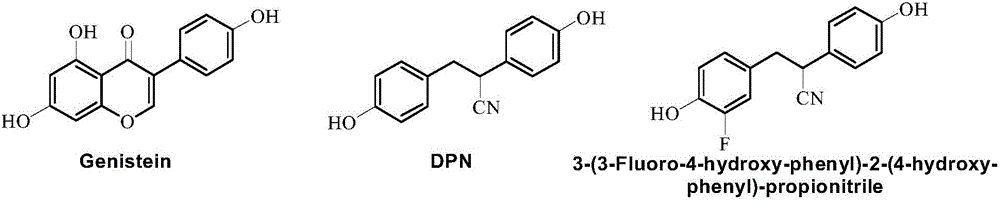
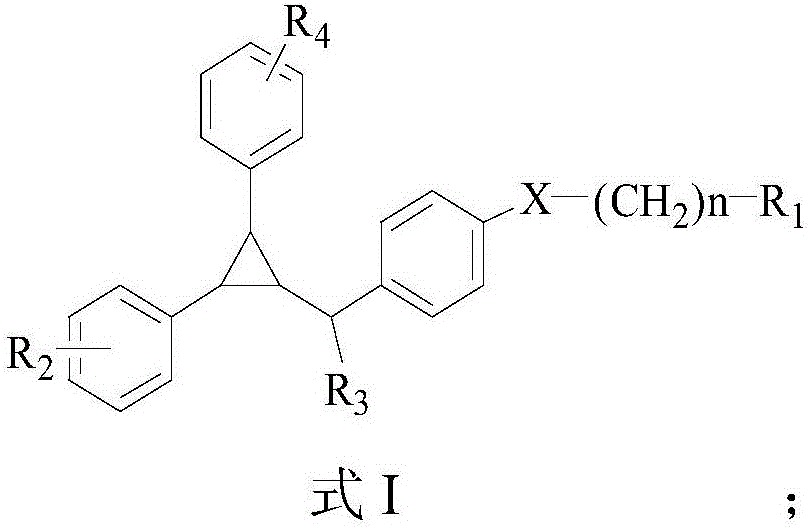
Cyclopropane compound as well as preparation method and application thereof
A technology of compound, cyclopropane, which is applied in the field of cyclopropane compounds and their preparation, to achieve the effects of wide sources, low cost and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1, Synthesis of 4-benzyloxybenzaldehyde
[0058]
[0059] Add 45g of 4-hydroxybenzaldehyde and 55g of potassium carbonate into a 500ml three-necked flask, perform anhydrous and oxygen-free operation, add 350ml of anhydrous acetone, and stir to dissolve. 52.3ml of benzyl bromide was added into the syringe, and the mixture was refluxed at 65°C for 8h. After the reaction was complete, distilled water was added to quench the reaction. The liquid was separated, the aqueous phase was extracted three times with Ea, the organic phase was washed once with water, dried by adding anhydrous sodium sulfate, concentrated to a small volume, and crystallized by adding Hex. 70.79 g of white crystals were obtained, with a yield of 90.88%.
[0060] 2. Synthesis of 1-(4-(3-chloropropoxy)phenyl)ethanone
[0061]
[0062] Add 34.04g of 4-hydroxyacetophenone and 41.37g of potassium carbonate into a 500ml three-neck flask, perform anhydrous and anaerobic operation, add 250ml of anhydr...
Embodiment 2
[0069] Synthesis of Example 2 (2-(4-(phenylmethoxy)phenyl)-3-phenylcyclopropyl) (4-(3-chloropropoxy)-phenyl)methanone
[0070]
[0071] The obtained (E)-3-(4-(benzyloxy)phenyl)-1-(4-(3-chloropropoxy)phenyl)propenone 19.90g and rhodium acetate 0.22g were added to 500ml three Neck bottle, carry out anhydrous and oxygen-free operation, add 200ml of toluene and stir to dissolve, add 5g of sulfurized cyclohexane, and slowly add 100ml of the toluene solution of diazomethylbenzene obtained dropwise through the constant pressure low liquid funnel, at room temperature 25°C The reaction was stirred for 5 days, and after the reaction was completed, the mixture was filtered through a floxacin funnel, washed with dichloromethane several times, and concentrated to dryness. Column purification, developer: petroleum ether: ether = 5:1. Dichloromethane:petroleum ether=2:1 for recrystallization. Obtained white solid: 7.49g, yield 30.83%.
[0072] The characterization data of this compound...
Embodiment 3
[0073] Example 3 (4-(3-(azetidinyl)propoxy)phenyl)(2-(4-(benzyloxy)phenyl)-3-phenylcyclopropyl)methanol (8a) Synthesis
[0074]
[0075] (2-(4-(Benzyloxy)phenyl)-3-phenylcyclopropyl)(4-(3-chloropropoxy)-phenyl)methanone 740mg, potassium carbonate 221.14mg, iodine Sodium chloride 27mg was placed in a 50ml three-necked bottle, and anhydrous and oxygen-free operation was carried out. 2 Add 5ml of freshly distilled acetonitrile under protection, stir to dissolve, add 0.16ml of arididine into the syringe, and react at 60°C for 24h, TLC (petroleum ether: ethyl acetate: triethylamine = 10:1:0.3%) monitors that the reaction is complete. to room temperature 25°C. Add 10ml of methanol, 32mg of sodium borohydride, stir the reaction at room temperature 25°C for 12h, TLC (petroleum ether: ethyl acetate: triethylamine = 2:1:0.3%) monitors the completion of the reaction, add ice water to quench under ice bath React, let it stand for half an hour, filter, wash the filter cake with disti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com