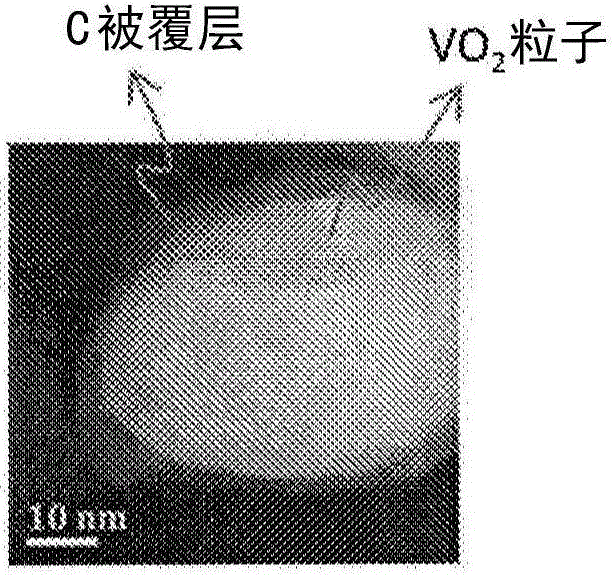
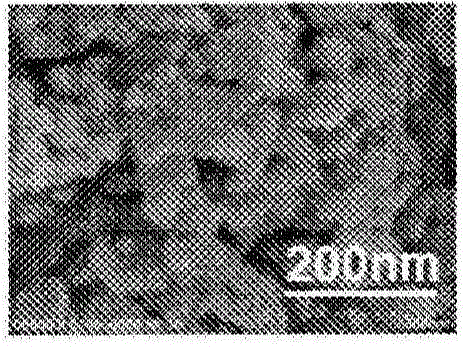
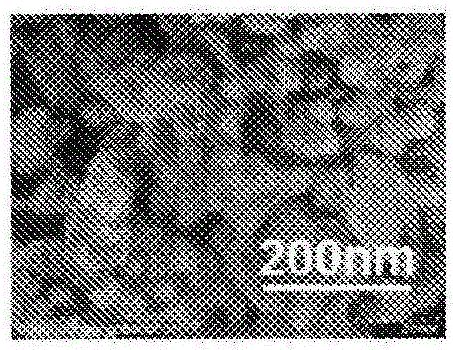Carbon-coated vanadium dioxide particles
A technology of vanadium dioxide particles and vanadium dioxide, which is applied in the direction of vanadium oxide, inorganic chemistry, tungsten compounds, etc., can solve the problems of inability to obtain particle size, small vanadium dioxide nanoparticles, etc., achieve excellent sintering inhibition effect, improve crystallization Sex, the effect of inhibiting oxidation or reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] (Production of vanadium dioxide particles)
[0123] Containing 1.299g of ammonium metavanadate (NH 4 VO 3 ) was slowly added dropwise to 4 ml of 10% hydrazine aqueous solution, and allowed to react at room temperature for 1 hour. Thereafter, the reaction liquid was transferred to a stainless steel pressure-resistant container with a fluorine-containing resin inner cylinder, and reacted at 270° C. for 48 hours. After the reaction, the particles were separated from the solution by centrifugation and washed three times. Afterwards, the particles were recovered by drying at 50°C.
[0124] Moreover, the particle diameter (volume average particle diameter) of the obtained vanadium dioxide particle was measured using the particle size distribution meter (Nikkiso Co., Ltd. make, MICROTRAC UAM-1).
[0125] (Formation of coating layer)
[0126] 0.1 g of 1,5-dihydroxynaphthalene (manufactured by Tokyo Chemical Industry Co., Ltd.), 0.05 g of 40% methylamine (manufactured by Wa...
Embodiment 2
[0133] Carbon-coated vanadium dioxide particles were produced in the same manner as in Example 1 except that vanadium dioxide particles were produced by the method shown below. In addition, in "(formation of coating layer)" of Example 1, "heating at 150 degreeC for 2 hours" was changed to "heating at 200 degreeC for 2 hours".
[0134] (Production of vanadium dioxide particles)
[0135] Containing 1.299g ammonium metavanadate (NH 4 VO 3 ) and 0.0329g ammonium tungstate hydrate ((NH 4 ) 10 W 12 o 41 ·5H 2O) 4.5 ml of 10% aqueous hydrazine solution was slowly added dropwise to 50 ml of the aqueous dispersion, and allowed to react at room temperature for 1 hour. Thereafter, the reaction solution was transferred to a stainless steel pressure vessel with a fluorine-containing resin inner cylinder, and reacted at 270° C. for 48 hours. After the reaction, the particles were separated from the solution by centrifugation and washed three times. Thereafter, the vanadium dioxide ...
Embodiment 3
[0138] Carbon-coated vanadium dioxide particles were produced in the same manner as in Example 1 except that the vanadium dioxide particles obtained in Example 2 were used to form a coating layer by the following method.
[0139] (Formation of coating layer)
[0140] 0.07 g of 1,5-dihydroxynaphthalene (manufactured by Tokyo Chemical Industry Co., Ltd.), 0.03 g of 40% methylamine (manufactured by Wako Pure Chemical Industries, Ltd.), and 0.07 g of 37% aqueous formaldehyde (manufactured by Wako Pure Chemical Industries, Ltd.) were sequentially dissolved in In ethanol, make 20g of ethanol mixed solution.
[0141] Next, 0.2 g of tungsten-doped vanadium dioxide particles were added to the obtained liquid mixture, and the mixture was treated in an ultrasonic bath for 6 hours. The solution was filtered, washed with ethanol three times, and vacuum-dried at 50° C. for 3 hours. Further, the dried particles were heated at 150° C. for 2 hours to obtain carbon-coated vanadium dioxide par...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com