Nanogel and nanogel drug carrier system both with smart response to tumor microenvironment
A nano-gel, responsive technology, applied in the field of polymer drug carriers, can solve the problems of slow pH responsiveness, time-consuming, and hindering anti-tumor effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
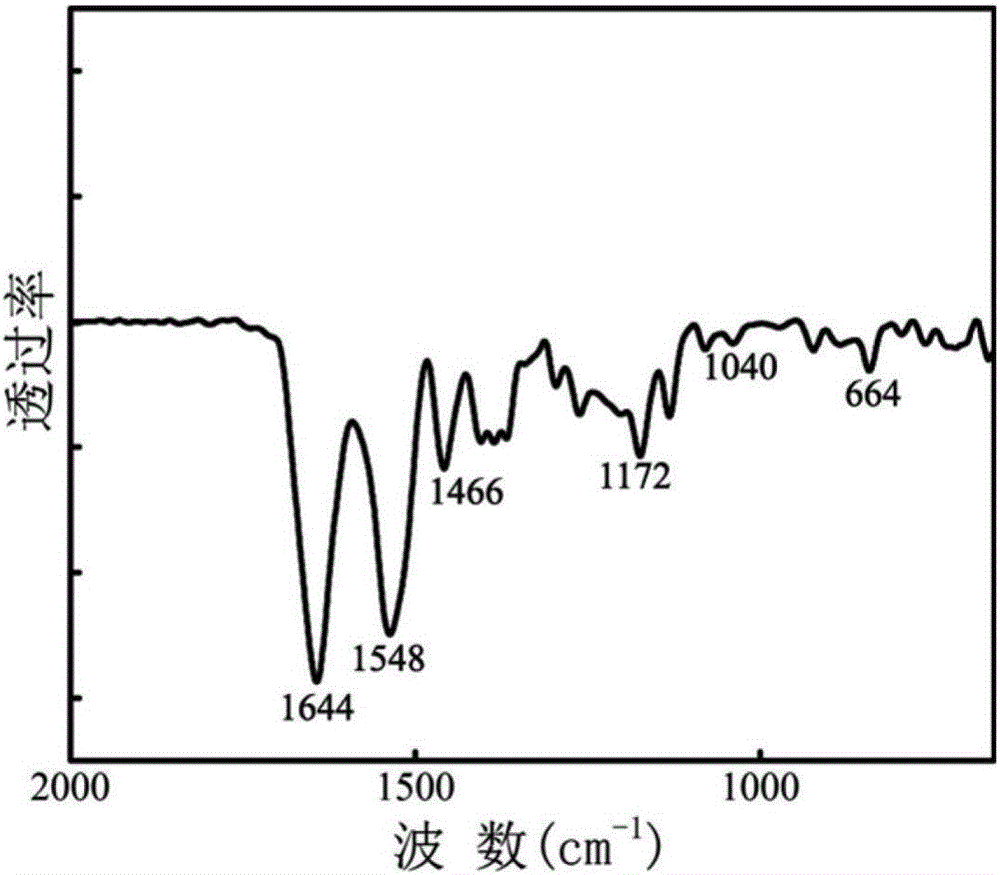
[0047] (1) Accurately weigh 1.908g N-isopropylacrylamide (NIPAM), 0.262g sulfobetaine methyl methacrylate (SBMA) and 0.18ml N-methallylamine (MAA) and dissolve in 500ml In a three-necked bottle, dissolve with 200ml of ultrapure water, add 0.04g of sodium lauryl sulfate as a surfactant and 0.098g of a cross-linking agent N,N'-bisacrylcystamine, and mix by ultrasonic. Corresponding to the molar ratio of N-isopropylacrylamide, sulfobetaine methyl methacrylate and N-methallylamine is 85.7:4.8:9.5, cross-linking of N,N'-bisacrylcystamine The density is 2 mol%.
[0048] (2) Pass nitrogen to the above reaction system to remove residual oxygen in the mixed solution. Heating in a water bath under magnetic heating stirring, slowly raising the temperature to 70-75°C, then adding 0.1g of potassium persulfate to initiate the polymerization reaction
[0049] (3) After the solution became turbid, the reaction was continued for 4.5 h at 70-75° C. under a nitrogen atmosphere.
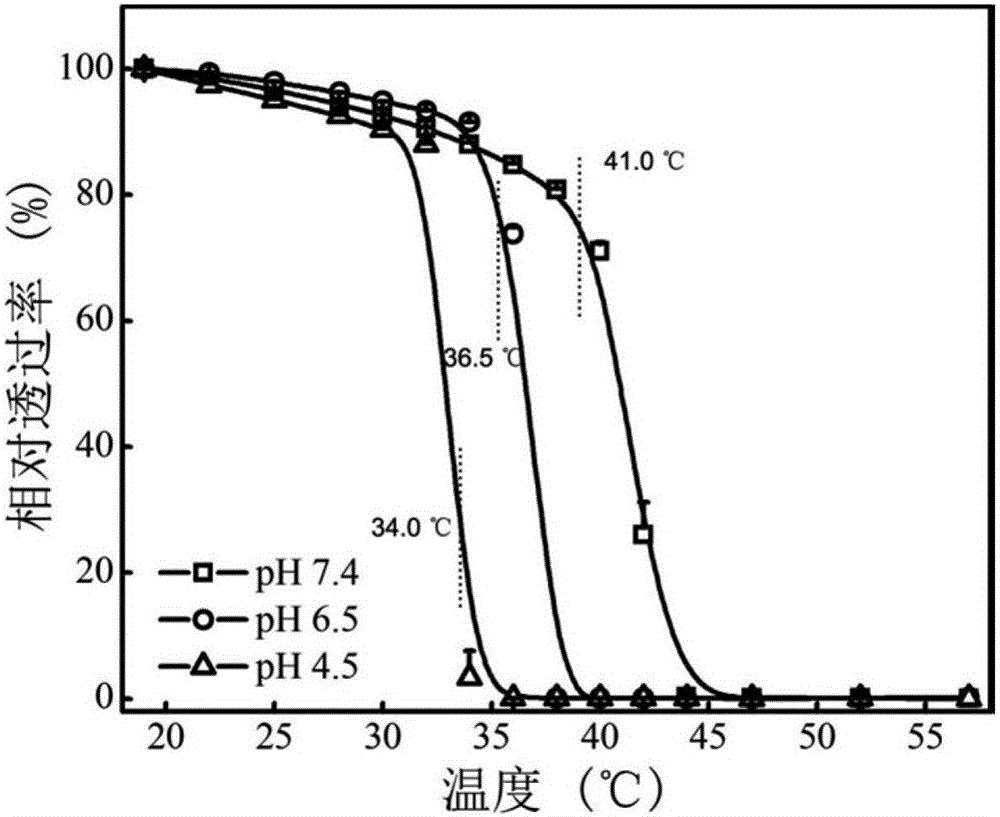
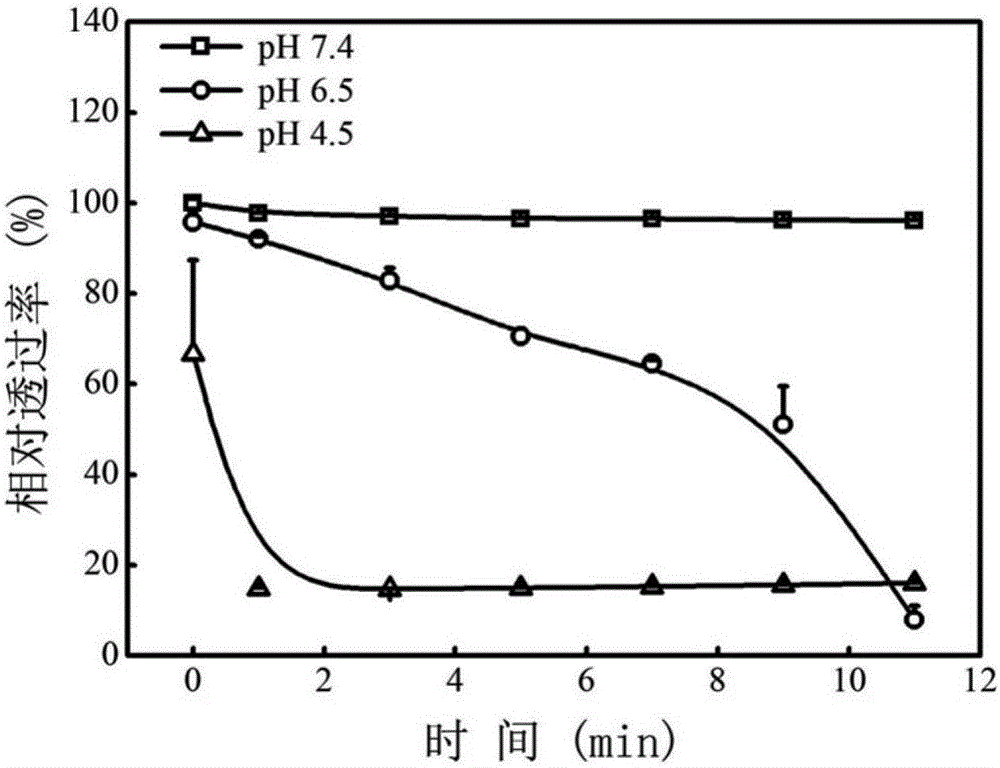
[0050] (4) T...
Embodiment 2
[0052] (1) Accurately weigh 2.012g of N-vinylisobutylamide, 0.250g of carboxybetaine methacrylate and 0.20g of methacryloyloxyethyltrimethylammonium chloride and dissolve them in a 500ml three-necked bottle , dissolved in 200ml ultrapure water, and added 0.04g sodium lauryl sulfate as a surfactant and 0.098g cross-linking agent N,N'-bisacryloylcystamine, and ultrasonically mixed. The molar ratio of N-vinylisobutylamide, carboxybetaine methacrylate and methacryloyloxyethyltrimethylammonium chloride is 89.6:5.5:4.9, N,N'-bisacryloyl Cystamine has a crosslink density of 1.9 mol%.
[0053] (2) Pass nitrogen to the above reaction system to remove residual oxygen in the mixed solution. Heating in a water bath under magnetic heating stirring, slowly raising the temperature to 70-75°C, then adding 0.1g of potassium persulfate to initiate the polymerization reaction
[0054] (3) After the solution became turbid, the reaction was continued for 4.5 h at 70-75° C. under a nitrogen atmos...
Embodiment 3
[0057] (1) Accurately weigh 1.739g of N-vinylcaprolactam, 0.434g of 2-methacryloyloxyethylphosphorylcholine and 0.202g of N,N'-dimethylallylamine and dissolve them in a 500ml three-necked bottle , dissolved in 200ml ultrapure water, and added 0.04g sodium lauryl sulfate as a surfactant and 0.05g crosslinking agent diallyl disulfide, and ultrasonically mixed. The molar ratio of N-vinylcaprolactam, 2-methacryloyloxyethylphosphorylcholine and N,N'-dimethylallylamine is 74.5:9.0:14.5, diallyl disulfide The crosslink density was 2.1 mol%.
[0058] (2) Pass nitrogen to the above reaction system to remove residual oxygen in the mixed solution. Heating in a water bath under magnetic heating stirring, slowly raising the temperature to 70-75°C, then adding 0.1g of potassium persulfate to initiate the polymerization reaction
[0059] (3) After the solution became turbid, the reaction was continued for 4.5 h at 70-75° C. under a nitrogen atmosphere.
[0060] (4) The obtained reaction s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface charge | aaaaa | aaaaa |
| Surface charge | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com