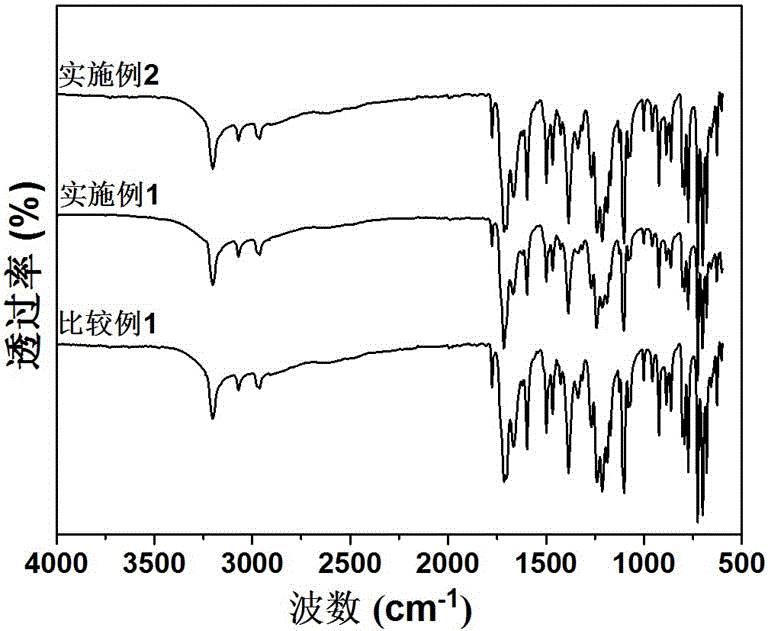
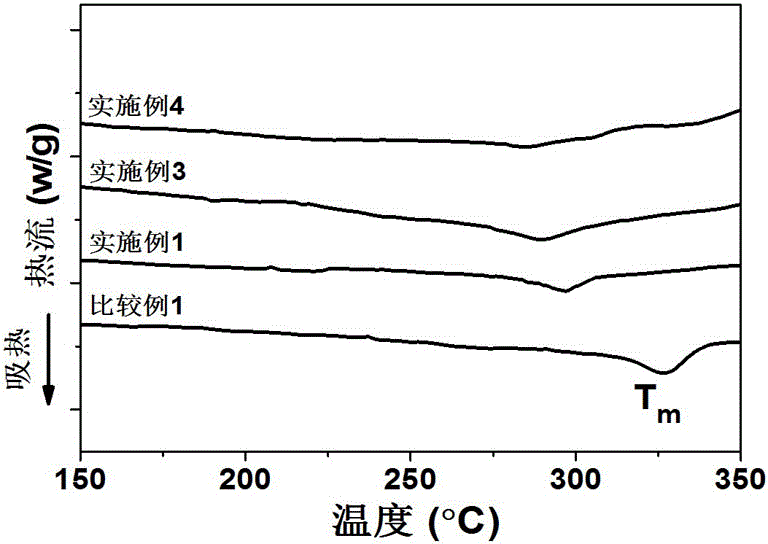
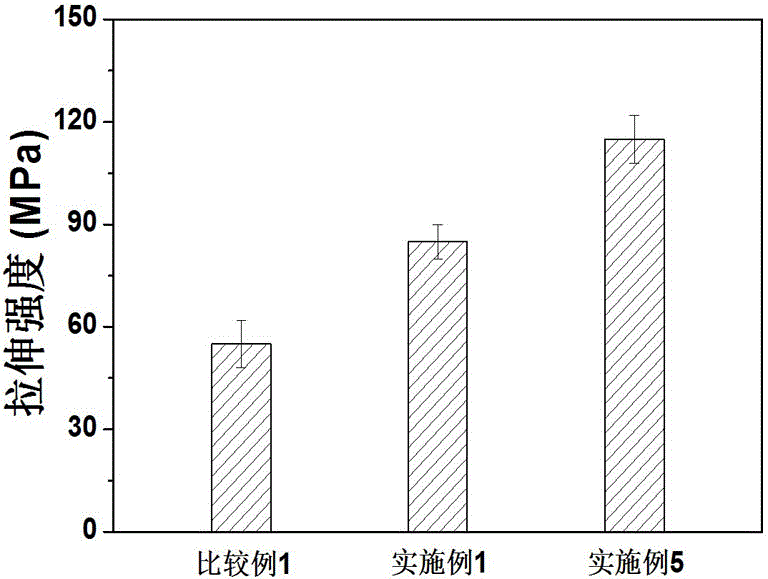
Aromatic polyesteramide and preparation method thereof
A polyester amide and aromatic technology, applied in the field of aromatic polyester amide and its preparation, can solve the problems that cannot meet the requirements of high temperature application fields, high melting point, difficult processing and molding, etc., to achieve outstanding heat resistance and moldability amount, self-reinforcing modulus, and the effect of lowering the melting point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In this example, polyester amide is prepared by using meta-aromatic diphenol, diacid monomer and semi-aromatic diamine monomer: add 110.11g 1,3-resorcinol, 332.26g 1,3-isophthalic acid, 136.20 g 1,4-xylylenediamine, 142 mL acetic anhydride, and 49.07 mg potassium acetate. The flask was fitted with a sealed glass paddle stirrer, a nitrogen inlet tube and an insulated distillation head. A moderate nitrogen flow was introduced, and the acetylation reaction was carried out at a temperature of 140° C. for 60 minutes. The reaction mixture was heated (0.5°C / min) in a quicksand bath, and the reaction temperature rose from 140°C to 310°C. At this point, the reaction system was slowly evacuated (vacuum degree: 10 mbar), and kept at 310° C. for 30 min. The opaque melt was cooled to room temperature and the product was removed from the flask and ground to a fine powder. Finish polycondensation in a vacuum oven with a vacuum degree of 10mbar at 250°C for 24 hours to obtain an aro...
Embodiment 2
[0032] In this example, polyester amide was prepared from meta-aromatic diphenol, diacid monomer and semi-aromatic diamine monomer: 186.21g of 1,1'-biphenyl-3,4 was added to a 1000mL three-neck round bottom flask '-diphenol, 605.58g 1,1'-biphenyl-3,3'-dicarboxylic acid, 372.62g 1,4-benzenedipentylamine, 189mL acetic anhydride and 82mg sodium acetate. The flask was fitted with a sealed glass paddle stirrer, a nitrogen inlet tube and an insulated distillation head. A moderate nitrogen flow was introduced, and the acetylation reaction was carried out at a temperature of 120° C. for 60 minutes. The reaction mixture was heated (0.8°C / min) in a quicksand bath, and the reaction temperature rose from 120°C to 300°C. At this point, the reaction system was slowly evacuated (vacuum degree: 1 mbar), and kept at 300° C. for 30 min. The opaque melt was cooled to room temperature and the product was removed from the flask and ground to a fine powder. In a vacuum oven at 240°C with a vacuu...
Embodiment 3
[0041] In this example, polyester amide was prepared from meta-aromatic diphenol, diacid monomer and semi-aromatic diamine monomer: 160.17g 1,7-naphthalenediol, 756.67g 1 , 7-naphthalene dicarboxylic acid, 676.03 g 1,4-naphthalene dibutylamine, 284 mL acetic anhydride, and 81.20 mg stannous octoate. The flask was fitted with a sealed glass paddle stirrer, a nitrogen inlet tube and an insulated distillation head. A moderate nitrogen flow was introduced, and the acetylation reaction was carried out at a temperature of 140° C. for 60 minutes. The reaction mixture was heated (1.0°C / min) in a quicksand bath, and the reaction temperature rose from 140°C to 320°C. At this point, the reaction system was slowly evacuated (vacuum degree: 5 mbar), and kept at 320° C. for 15 min. The opaque melt was cooled to room temperature and the product was removed from the flask and ground to a fine powder. Finish polycondensation in a vacuum oven with a vacuum degree of 5mbar at 230°C for 24 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com