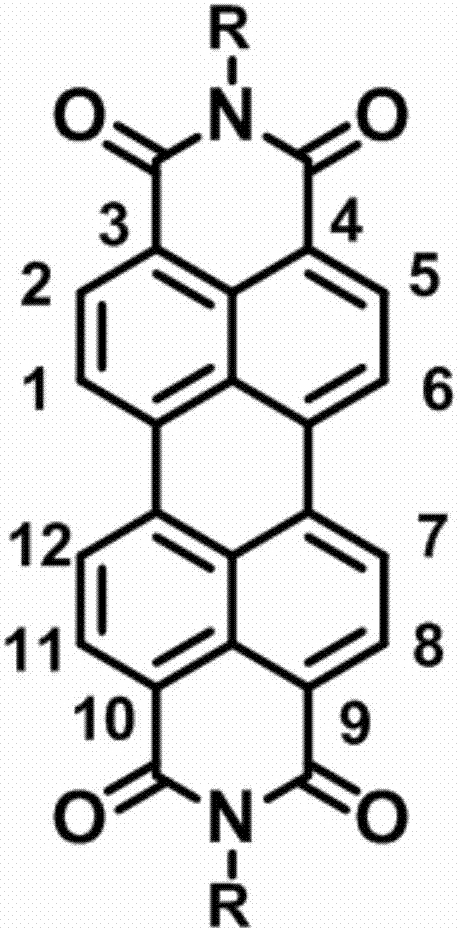
Perylene diimide organic third-order nonlinear optical material, and preparation and application of perylene diimide organic third-order nonlinear optical material
A third-order nonlinear, perylene imide-based technology, applied in nonlinear optics, luminescent materials, organic chemistry, etc., to achieve the effects of easy operation, large nonlinear absorption coefficient, and high two-photon absorption characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of a peryleneimide-based organic nonlinear optical material mainly includes the following steps:
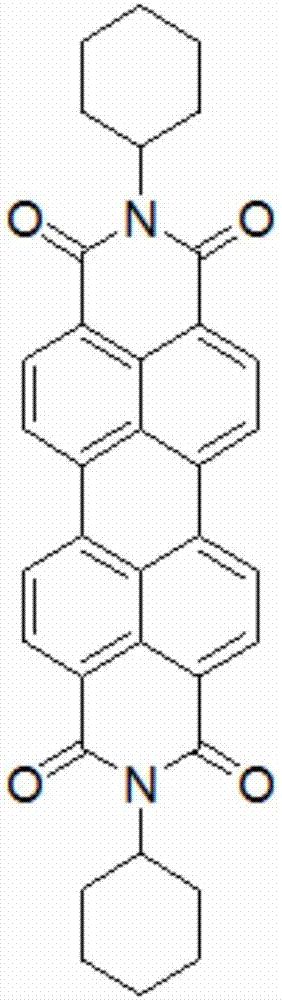
[0035] First prepare the perylene imide derivatives substituted by cyclohexane, the reaction formula is as follows:
[0036]
[0037] Then use the peryleneimide derivatives substituted by cyclohexane to react with the benzoanthrone derivatives that introduce boric acid pinacol ester at the 3rd position, and the reaction formula is as follows:
[0038]
[0039] It mainly includes the following steps: take the peryleneimide derivative (500mg, 0.76mmol) substituted by cyclohexane, introduce the benzanthrone derivative (315mg, 0.92mmol) of boric acid pinacol ester at the 3rd position and K 2 CO 3 (133mg), dissolved with toluene-ethanol mixed solution (volume ratio 3:1, 80mL). After passing nitrogen gas for 30 minutes, add 15 mg tetrakistriphenylphosphine palladium catalyst to the solvent, heat to 80° C., 1000 r / min, and react for 5 hours. Cool to room ...
Embodiment 2
[0042] A kind of preparation of perylene imide-based organic nonlinear optical material, its reaction formula is as follows:
[0043]
[0044]It mainly includes the following steps: take compound A (92mg, 0.12mmol) and dissolve it in 100ml of dichloromethane, transfer it to a quartz beaker, and place it under outdoor sunlight. The progress of the light reaction was observed by thin-layer chromatography. Due to the poor solubility of compound B, compound B after light-closing rings formed flocs, which were suspended or sank at the bottom of the beaker. The ring-closed Compound B was collected by filtration through micropores. Compound B was obtained after 24 hours as Figure 4 As shown, 3,4,9,10-perylenetetracarboximide is used as the core group, and cyclohexane and benzanthrone are used as substituent groups; two cyclohexanes are respectively connected at 3,4 ,9,10-Perylenetetracarboximide is fused to the bay position of 3,4,9,10-perylenetetracarboximide on the imide nitr...
Embodiment 3
[0047] A kind of preparation of perylene imide-based organic nonlinear optical material, the reaction formula is as follows:
[0048]
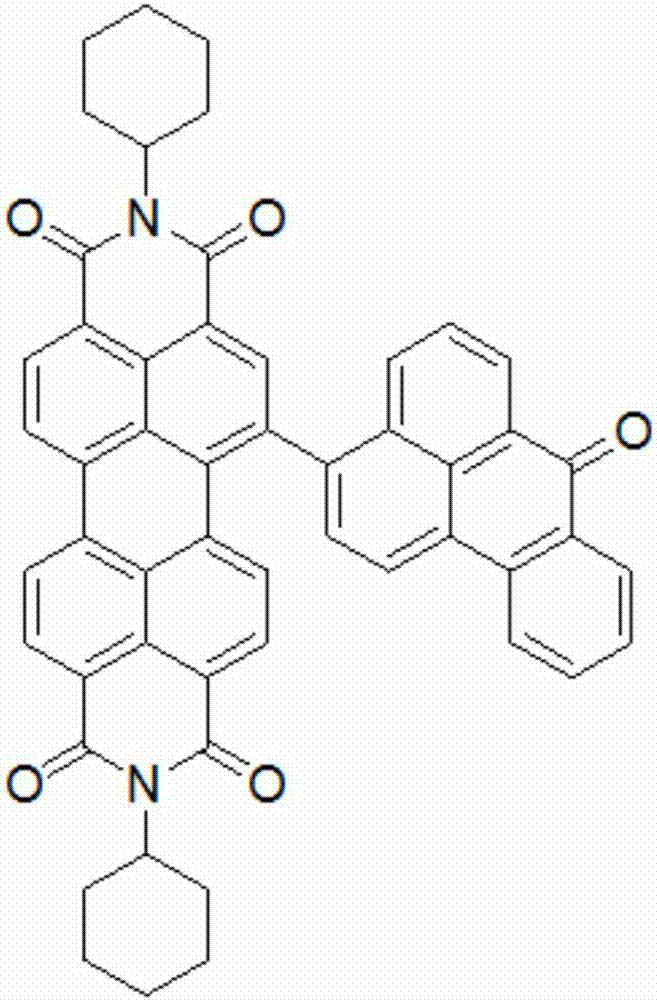
[0049] It mainly includes the following steps: taking perylene imide derivatives (0.356g, 0.5mmol) and triphenylamine derivatives (0.445g, 1.2mmol) and dissolving them in 40ml of tetrahydrofuran. Add 20ml of 2M potassium carbonate solution, treat with nitrogen for 30min, add tetrakistriphenylphosphine palladium catalyst 15mg, heat to 80°C, 1000r / min, and react for 24 hours. After cooling, it was extracted several times with dichloromethane and water, and the organic phase was removed with anhydrous sodium sulfate, separated, and dried. The crude product was separated and purified with a 200-300 mesh silica gel column, dichloromethane-n-hexane (volume ratio 1:3) mixed solvent eluent, to obtain compound C such as Figure 5 As shown, 3,4,9,10-perylenetetracarboximide is used as the core group, and cyclohexane and triphenylamine are used as su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com