Drug composition for treating multiple sclerosis
A technology of gel composition and swollen product, which is applied in drug combination, drug delivery, and pharmaceutical formulation, etc. It can solve problems such as secretion and swallowing affecting the effectiveness of oral mucosal pathways, drug taste stimulation affecting compliance, and poor control of drug doses, etc. , to avoid the first-pass effect of the liver, to facilitate self-medication, and to improve skin and mucous membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
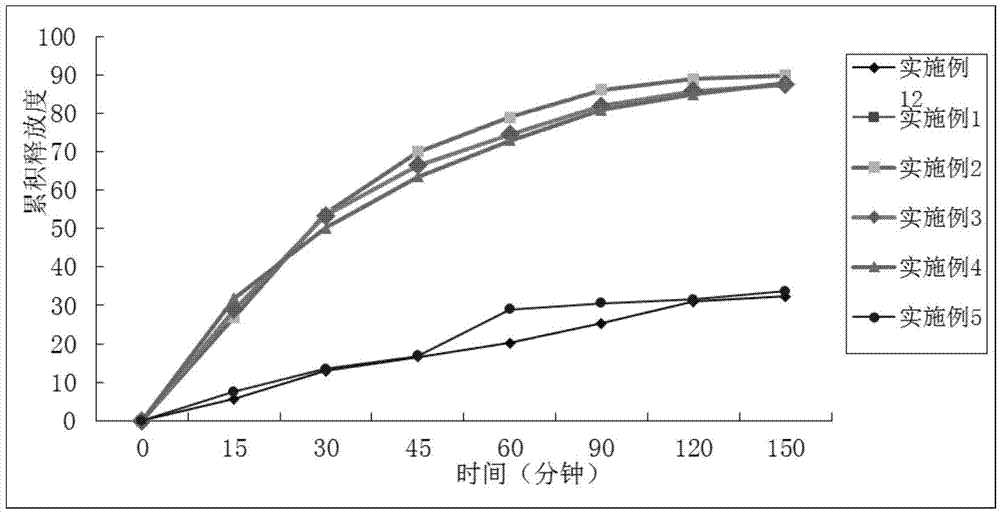
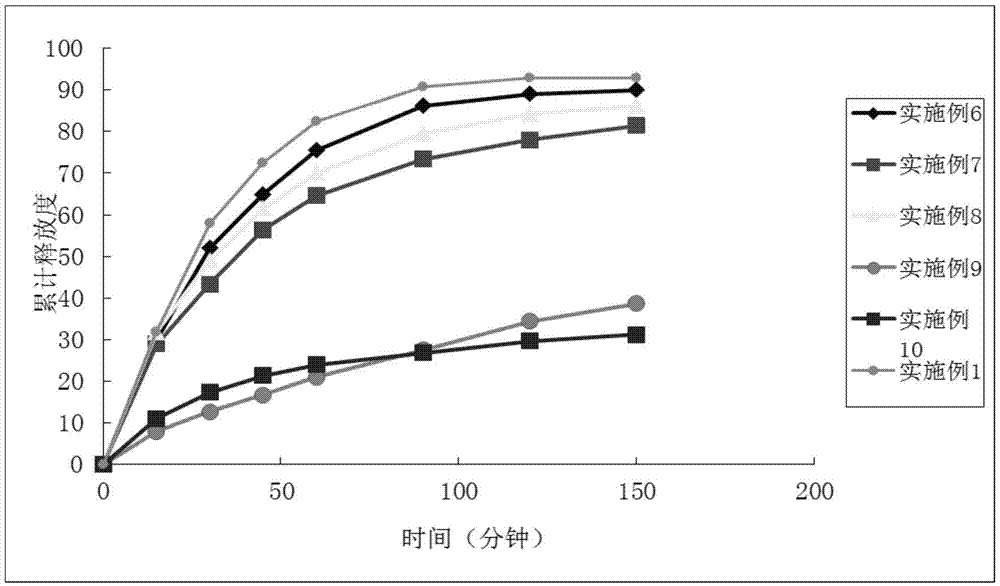
Examples
Embodiment 1
[0049] Glatiramer Acetate Nasal Gel Composition:
[0050]
[0051] The preparation steps of the gel preparation of every 200 grams are:
[0052] (1) Take the gel matrix carbomer of the prescribed amount, add 3 to 5 times the weight of water, stir to make it fully swell, add dropwise sodium hydroxide to adjust the pH value to 7.0 to 8.0, and stir to obtain a gel;
[0053] (2) Separately take the prescribed amount of glatiramer acetate plus 2 to 5 times the weight of water and dissolve it;
[0054] (3) Stir the dextran, glucose, glycerin and EDTA with 2 to 3 times the weight of water to dissolve them;
[0055] (4) Add the solution of step (2) and step (3) to the gel matrix solution of step (1), then add the absorption enhancer 2-hydroxypropyl-β-cyclodextrin, stir well, and then add water to 200g, and stir evenly to obtain the finished gel.
Embodiment 2
[0057]
[0058] The preparation steps of the gel preparation of every 200 grams are:
[0059] (1) Take the gel matrix polyvinyl alcohol of the prescribed amount, add 4 to 7 times the weight of water, add dropwise acetic acid to adjust the pH value to 5.0 to 6.0, and stir to make it fully swell;
[0060] (2) Separately take the prescribed amount of glatiramer acetate plus 2 to 5 times the weight of water and dissolve it;
[0061] (3) Add mannitol, benzalkonium chloride, propylene glycol, and chitin to 1 to 2 times the weight of water and stir to dissolve them;
[0062] (4) Add the solution of step (2) and step (3) to the gel matrix solution of step (1), then add the absorption-promoting agent lecithin, stir evenly, then add water to 200g, stir evenly, and obtain Finished gel. Example 3:
Embodiment 3
[0063] Glatiramer Acetate Nasal Gel Composition:
[0064]
[0065] The preparation steps of the gel preparation of every 200 grams are:
[0066] (1) Weigh the hypromellose of prescription quantity, add the water of 4~7 times by weight, stir while adding, make it fully swell;
[0067] (2) Separately take the prescribed amount of glatiramer acetate plus 2 to 5 times the weight of water and dissolve it;
[0068] (3) Stir sodium chloride, EDTA, glycerin and starch with 3 to 4 times the weight of water to dissolve them;
[0069] (4) Add the solution of step (2) and step (3) to the gel matrix solution of step (1), then add the absorption-promoting agent sodium glycocholate, stir well, then add water to 200g, stir well, that is A finished gel is produced.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com