Hydrogen-peroxide-enhanced fluorescent probe based on Rhodamine derivatives
A fluorescent probe and hydrogen peroxide technology, applied in the field of analytical chemistry, can solve the problems of being unsuitable for small animals, short excitation wavelength, etc., and achieve the effects of simple synthesis process, rapid detection, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
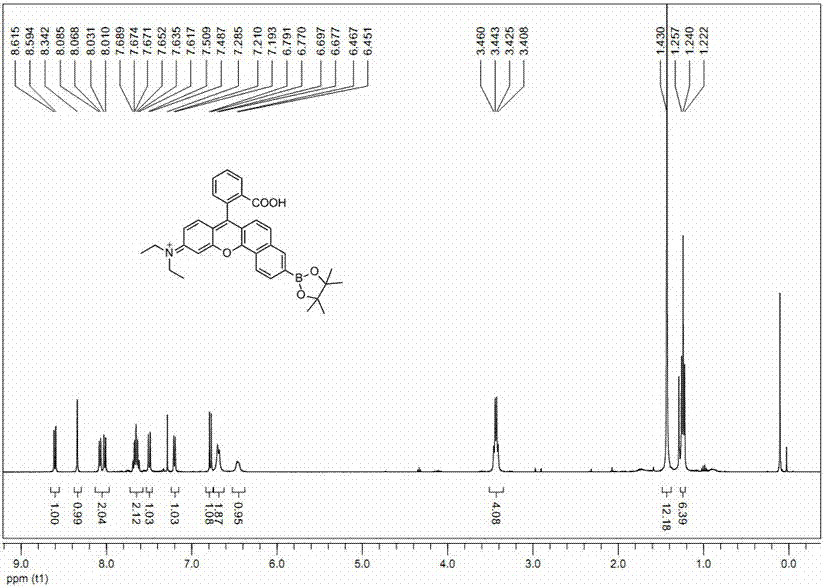
[0028] Preparation of fluorescent probes:
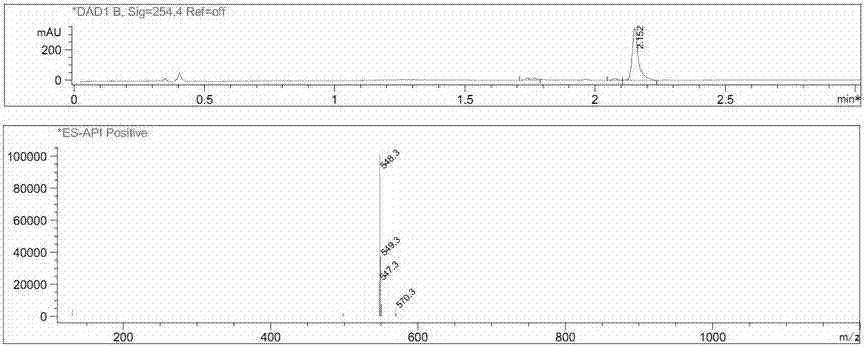
[0029] Dissolve intermediate 1 (1 mmol), N,N-bis(trifluoromethanesulfonyl)aniline (1 mmol) and N,N-diisopropylethylamine (2 mmol) in 5 ml DMF and react 6 at room temperature After 1 hour, 5 ml of water was added, extracted with 5 ml of ethyl acetate, and the oil phase was vacuum-dried to obtain a crude product. The crude product was purified by column chromatography (eluent: ethyl acetate:petroleum ether = 1:5) to obtain the pure intermediate 2 (yield 73%). Intermediate 2 (1 mmol), bis-linked pinacol borate (1 mmol), 1,1-bis(diphenylphosphino)ferrocene dichloropalladium dichloromethane complex (0.2 mmol) and potassium acetate (1.5 mmol) were dissolved in 10 ml of toluene, and refluxed for 6 h under nitrogen protection. After the reaction, the solvent was removed in vacuo to obtain a crude product. The crude product was purified by column chromatography (eluent: ethyl acetate:petroleum ether = 1:4) to obtain a pure probe (yield 69%...
Embodiment 2
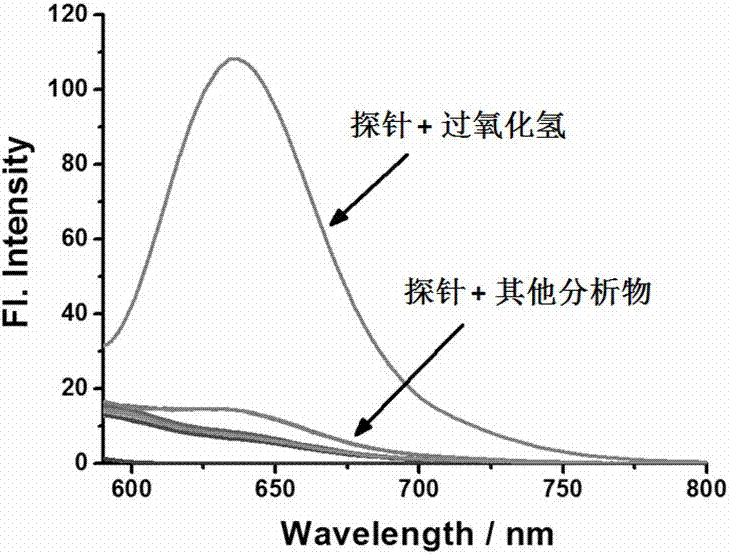
[0031] Selective Identification of Different Substances by Fluorescent Probes
[0032] Prepare 10 parts of 5 mL of 5 μM fluorescent probe PBS solution (containing 5% methanol) in advance, and then add 50 μL of 100 μM hydrogen peroxide, sodium hypochlorite, nitric oxide, semi Cystine, hydroxyl radical, di-tert-butyl peroxide, di-tert-butanol peroxide, glutathione, vitamin C and sodium nitrate in PBS. Perform fluorescence detection (λ Ex = 580 nm); calculate the fluorescence intensity I at 638 nm in each system 638 , see the result image 3 . It shows that the fluorescent probe has better selectivity to hydrogen peroxide.
Embodiment 3
[0034] Linear relationship between fluorescent probe and hydrogen peroxide
[0035] Prepare 1 mL of PBS solution system of hydrogen peroxide with a gradient concentration, and then add 9 mL of probe PBS solution (containing 5% methanol) with a concentration of 50 μM respectively for fluorescence detection (λ Ex = 580 nm, λ Em = 638 nm), calculate the fluorescence intensity in each system, and the fluorescence intensity I at 638 nm 638 , to establish a standard curve of fluorescence intensity and hydrogen peroxide concentration, the standard curve is shown in Figure 4 . The detection limit of the fluorescent probe of the present invention is 0.071 μM (S / N = 3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com