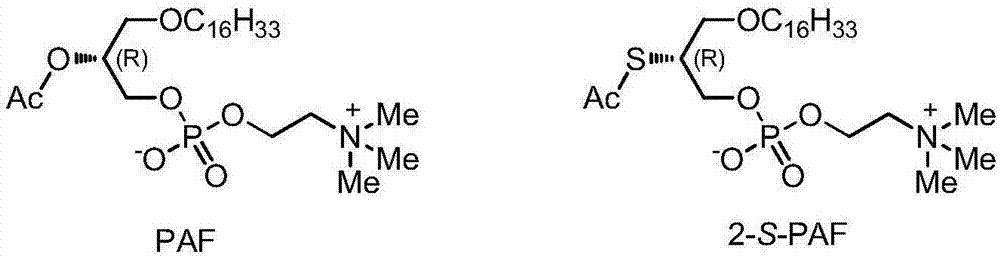
Platelet activating factor derivative and synthesis method thereof
A technology of activating factor and synthetic method, applied in the preparation of 2-thio fatty chain carboxylic acid platelet activating factor and its derivatives, in the field of platelet activating factor derivatives, can solve the problem of unstable yield and low 2-S-PAF , limit the market application of 2-S-PAF and other issues, and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the synthesis of 2-S-PAF
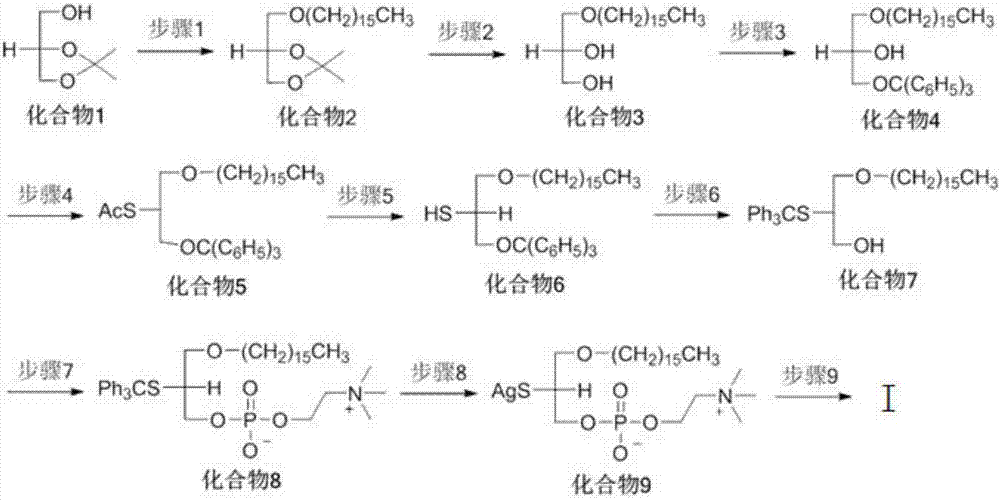
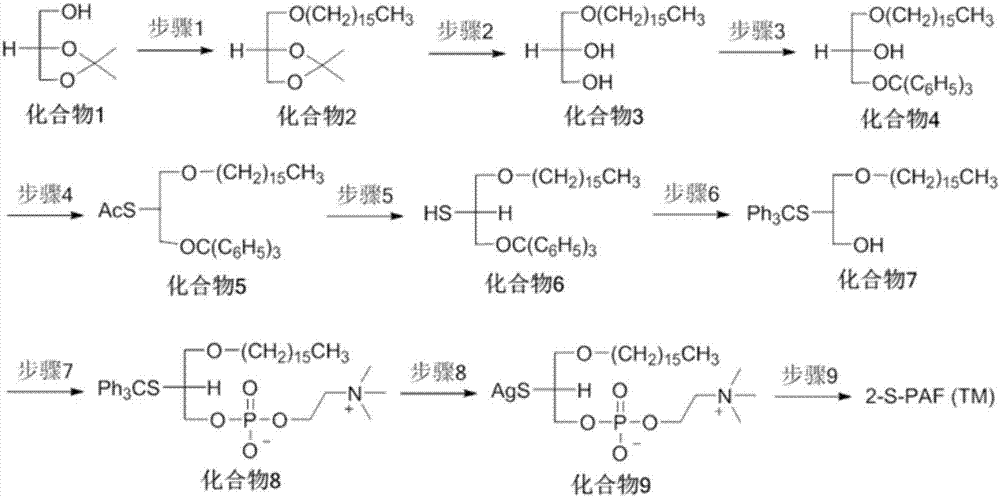
[0038] This embodiment provides the synthetic route figure of 2-S-PAF, see for details image 3 . The final product 2-S-PAF is denoted by TM.
[0039] In the synthetic route given in this example, the specific operation of step 1 is to add 300 mL of dimethylformamide (DMF) solution dissolved with NaH (7.26 g, 0.182 mol) in a round bottom flask, and then add compound 1 (20.0 g, 0.151mol), stirred for 30min; then hexadecylsulfonate (58.21g, 0.182mol) was added to the reaction solution, and reacted at room temperature for 12 hours; the reaction solution was processed, extracted to obtain an organic phase, and silica gel column purification (elution The solvent is n-hexane / ethyl acetate, v / v=95 / 5) to obtain 39.0 g of compound 2 with a yield of 85%.
[0040] 1 H NMR (CDCl 3 )δ0.88(t,J=6.4Hz,6H),1.30(m,28H),1.56(m,5H),3.63(m,5H).
[0041] The specific operation of step 2 is that compound 2 (52.37g, 0.147mol) is dissolved in 370mL...
Embodiment 2
[0060] Embodiment 2, the synthesis of a kind of 2-S-PAF derivative
[0061] The synthetic route given in this embodiment can be found in image 3 , see embodiment 1 for the specific operation of steps 1-8.
[0062] The specific operation of step 9 is to dissolve compound 9 (30mg, 0.05mmol) in 2mL of MeCN, add KI (8.3mg, 0.05mmol), DMAP (6mg, 0.05mmol) and hexadecanoyl chloride (137mg, 0.5mmol ), reacted for 16-24h to obtain the product. with CHCl 3 / MeOH as the eluent, separated and purified by column chromatography to obtain 2-S-PAF derivatives with a yield of 61%.
[0063] 1 H NMR (CDCl 3 )δ0.88(t, J=6.4Hz, 3H), 0.89(t, J=6.4Hz, 3H), 0.91(t, J=6.4Hz, 3H), 1.20-1.42(m, 64H), 1.42- 1.60(m,2H),1.60-1.80(m,2H),1.96-2.18(m,4H),2.33(t,J=7.6Hz,2H),2.54(t,J=7.6Hz,2H),2.72 -2.92(m,6H),3.30-3.47(m,2H),3.40(s,9H),3.48-3.68(m,2H),3.75-4.03(m,5H),4.26-4.42(m,2H) ,5.24-5.50(m,8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com