Modifiable second near-infrared window fluorescent imaging probe and preparation method and application thereof
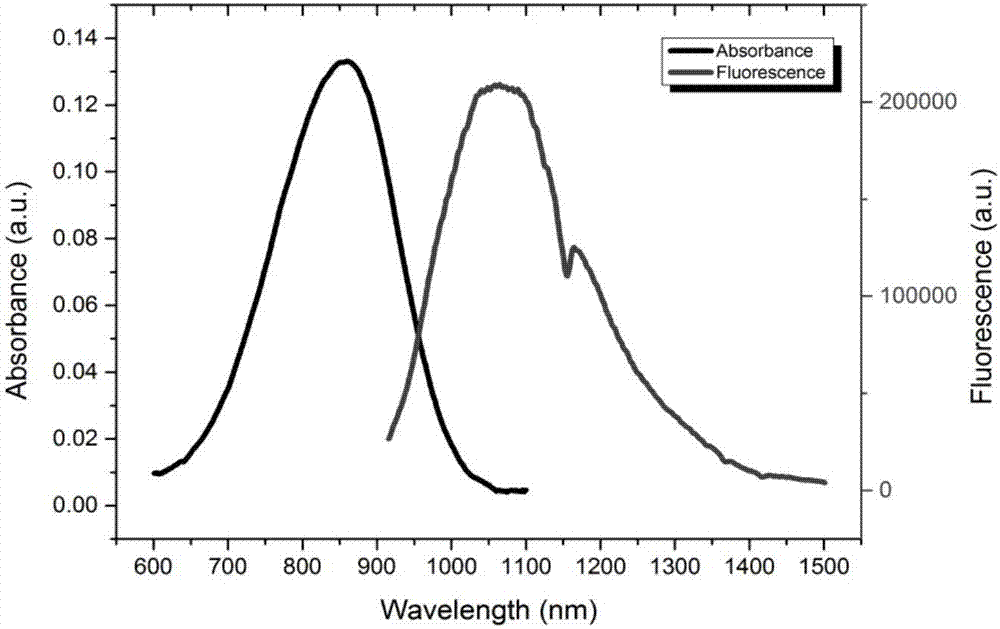
A technology of fluorescent compounds and dichloromethane, which is applied in the fields of biomedical fluorescence imaging and biomedical materials, can solve problems such as interference with fluorescence imaging effects, and achieve broad application prospects, high yield, and the effect of increasing quantum yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
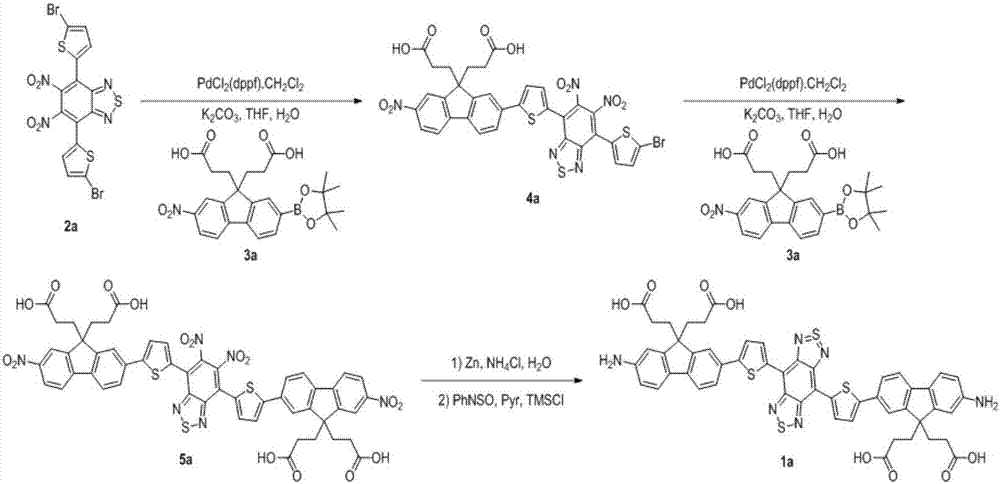
[0039] Embodiment 1: the preparation of compound 4a
[0040] Take compound 2a (5.48g, 10mmol), compound 3a (4.81g, 10mmol) and potassium carbonate (3.45g, 25mmol) into a 500mL round bottom flask, add tetrahydrofuran-water (v / v, 5: 1) 300mL, feed argon into the reaction solution and bubble for 5min to get rid of oxygen in the system, add [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex (0.9 g, 1 mmol), heated to reflux in an oil bath at 75° C. for 14 hours under the protection of argon. After the reaction, cool to room temperature, remove THF by rotary evaporation, redissolve the residue in 200 mL of dichloromethane, wash with water (50 mL×3) three times, and wash with saturated brine (50 mL×3) three times. The organic phase was dried with anhydrous magnesium sulfate for 3 hours, filtered, and the filtrate was spin-dried to obtain 7.4 g of compound 4a. Yield: 90%.
[0041] The structure determination data of compound 4a are as follows:
[0042...
Embodiment 2
[0043] Embodiment 2: the preparation of compound 5a
[0044] Take compound 4a (7.4g, 9mmol), compound 3a (4.329g, 9mmol) and potassium carbonate (3.105g, 22.5mmol) into a 500mL round bottom flask, add tetrahydrofuran-water (v / v, 5 : 1) 270mL, in the reaction solution, pass into argon bubble 5min and get rid of oxygen in the system, add [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium dichloromethane complex ( 0.81g, 0.9mmol), under the protection of argon, heated to reflux in an oil bath at 75°C for 20 hours. After the reaction, cool to room temperature, remove tetrahydrofuran by rotary evaporation, redissolve the residue in 150mL dichloromethane, wash with water (30mL×3) three times, and wash with saturated brine (30mL×3) three times. The organic phase was dried with anhydrous magnesium sulfate for 3 hours, filtered, and the filtrate was spin-dried to obtain 9.08 g of compound 5a. Yield: 92%.
[0045] The structure determination data of compound 5a are as follows: ...
Embodiment 3
[0049] Embodiment 3: the preparation of compound 1a
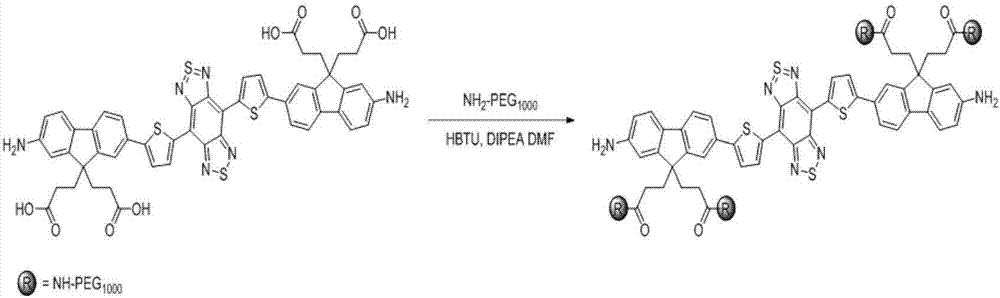
[0050] Take compound 5a (9.08g, 8.28mmol), zinc powder (64.915g, 993.6mmol) and ammonium chloride (15.947g, 298.08mmol) into a 1000mL round bottom flask, add dichloromethane 300mL and methanol -Water (v / v, 9:1) 300mL mixed solvent, the reaction solution was mechanically stirred at 25°C for 4 hours. After the reaction was finished, filter through diatomaceous earth to remove insoluble solids, add 300mL dichloromethane to wash, collect the filtrate, wash three times with water (100mL×3), wash three times with saturated sodium bicarbonate solution (100mL×3), wash with saturated saline (100mL×3) After washing three times, the organic phase was dried with anhydrous magnesium sulfate for 3 hours, filtered, and the solvent was removed to obtain an intermediate. Under argon protection in a 250mL round bottom flask, the intermediate was added to 160mL of anhydrous pyridine, N-sulfinanilide (9.09g, 66.24mmol) and trimethylchlorosila...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com