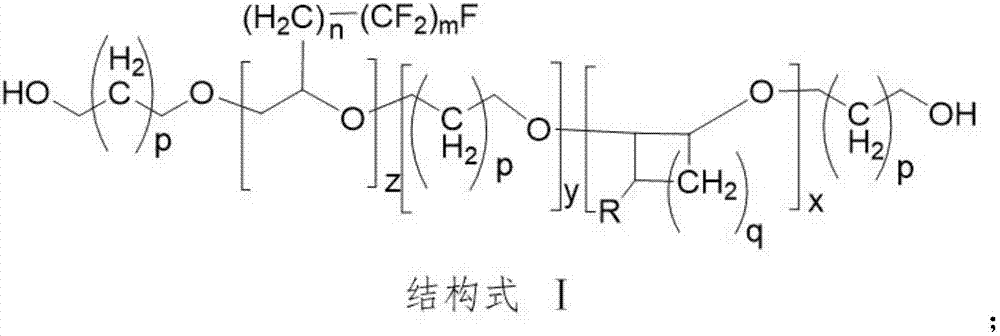
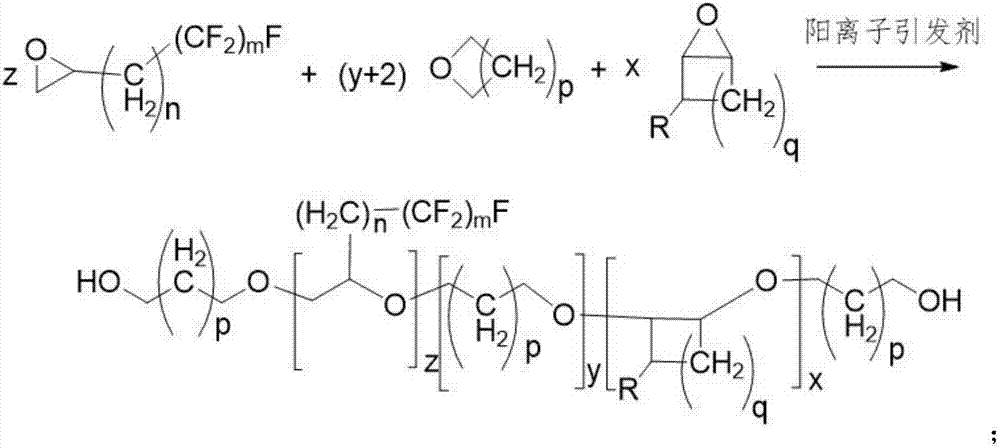
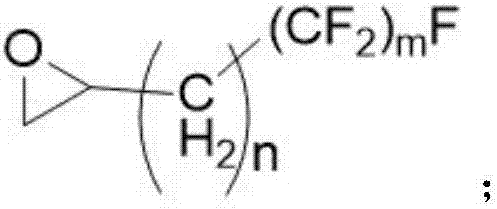
Diffluent side-chain fluorine-containing polyether diol prepared from perfluorinated ethylene oxide and multicomponent cyclic ether through copolymerization
A technology of copolyether diol and ethylene oxide, which is applied in the field of soluble side chain fluorine-containing polyether diol and its preparation, can solve the problems of rising use cost, waste of effective resources, harsh solvents, etc., and reduce processing cost , expand the use of space, and reduce pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Dissolve 0.05 moles of 2-(heptafluorotetradecyl)oxirane and 0.95 moles of 2-methylphenyl glycidyl ether in 100 mL of dichloromethane to form a dichloromethane solution of a three-membered cyclic ether .
[0037]Then, add 5 moles of oxetane, 50 mL of dichloromethane, 0.15 moles of 98% concentrated sulfuric acid and 0.18 moles of ethylene glycol into a 1000 mL kettle, cool down to -10 ° C, stir for 20 minutes and maintain this temperature, drop Add 0.05 moles of 2-(heptafluorotetradecyl)oxirane and 0.95 moles of 2-methylphenyl glycidyl ether in dichloromethane solution, and control the dripping within 2 hours. Maintain at -10°C and react for 10 hours. Add 20ml of deionized water to terminate the reaction, distill off the solvent, and neutralize to neutral with sodium bicarbonate solution. Add 200 mL of deionized water, stir and wash with water for 20 minutes, let stand to separate layers, remove the solvent from the oil phase by rotary evaporator, and obtain crude polye...
Embodiment 2
[0039] Dissolve 0.1 mol of 2-(heptadecafluorononyl)oxirane and 0.5 mol of benzyl glycidyl ether in 200 mL of ether to form an ether solution.
[0040] In a 1000mL kettle equipped with a stirrer, after replacing the air in the kettle with pure nitrogen, cool down to -5°C and add 100mL of ether as a solvent, add 1.8 moles of oxetane, 0.072 moles of perchloric acid and 0.12 moles of butane alcohol. The ether solution formed by dissolving 0.1 moles of 2-(heptadecafluorononyl)oxirane and 0.5 moles of benzyl glycidyl ether and 200 mL of diethyl ether was added dropwise, and the controlled dropping was completed within 3 hours. Keep at -5°C, react for 8 hours, add 30ml of deionized water to terminate the reaction, distill off the solvent, and neutralize to neutral with sodium carbonate solution. Add 200 mL of deionized water to wash for 20 minutes, let stand to separate, and wash the oil phase with distilled water again, let stand to separate. The oil phase was then vacuum-dried at...
Embodiment 3
[0042] Dissolve 0.15 mol of 2-perfluoropentyl oxirane and 0.3 mol of benzyl glycidyl ether together in 250 mL of acetone to form a three-membered cyclic ether in acetone.
[0043] In a 1000 mL kettle equipped with a stirrer, after replacing the air in the kettle with pure nitrogen, cool down to -0°C and add 100 mL of acetone as a solvent, add 0.45 moles of tetrahydrofuran, 0.0225 moles of perchloric acid and 0.027 moles of butanediol. The above-mentioned acetone solution formed by dissolving 0.15 moles of 2-perfluoropentyl oxirane and 0.3 moles of benzyl glycidyl ether in 250 mL of acetone was added dropwise, and the controlled dropping was completed within 3 hours. Keep at 0°C, react for 10 hours, add 50ml of deionized water to terminate the reaction, distill off the solvent, and neutralize to neutral with ammonium carbonate aqueous solution. Add 200 mL of deionized water to wash for 20 minutes, let stand to separate, and wash the oil phase with distilled water again, let sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com