Polyethylene glycol electrolyte oral liquid and preparation method thereof
A technology of polyethylene glycol and oral liquid, which is applied in the field of medicine, can solve the problems of affecting the solubility of polyethylene glycol, easy adsorption of drug powder, inconvenient storage and transportation, etc., to achieve convenient taking, storage and transportation, better clinical compliance, good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
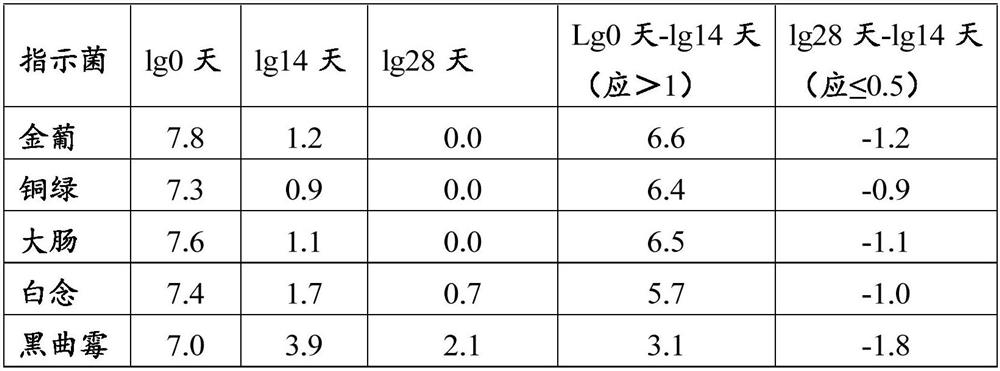
[0034] 1. Weigh 525g of polyethylene glycol 4000, 14.028g of sodium chloride, 1.864g of potassium chloride, 7.14g of sodium bicarbonate, appropriate amount of deionized water, set aside
[0035] 2. Take an appropriate amount of deionized water, heat it to 40°C, add 14.028g of sodium chloride, 1.864g of potassium chloride, and 7.14g of sodium bicarbonate, and stir to dissolve it completely
[0036] 3. Add polyethylene glycol 4000 525g to the above 2) solution, stir to dissolve completely, add water to 1000ml constant volume, stir to form a uniform and stable solution, put them into 500ml and 25ml containers respectively, stopper and seal Just save it.
[0037] After determination, its dynamic viscosity at room temperature is 128mpa.s.
Embodiment 2
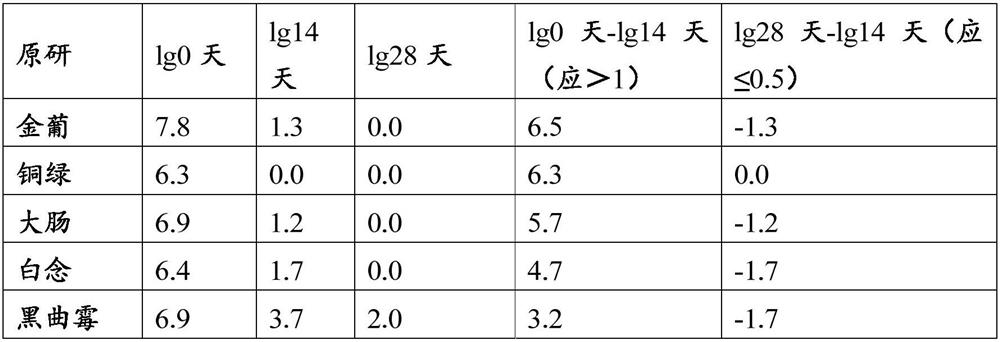
[0039]1. Weigh 291g of polyethylene glycol 3000, 28.056g of sodium chloride, 3.728g of potassium chloride, 14.28g of sodium bicarbonate, 0.3g of aspartame, appropriate amount of deionized water, set aside
[0040] 2. Take an appropriate amount of deionized water, 28.056g of sodium chloride, 3.728g of potassium chloride, 14.28g of sodium bicarbonate, and 0.3g of aspartame, stir to dissolve completely, and set aside.
[0041] 3. Add polyethylene glycol 3000 291g to the above 2) solution, stir to dissolve completely, add water to 1000ml constant volume, stir to form a uniform and stable solution, put them into 500ml and 25ml containers respectively, stopper and seal Just save it.
[0042] After determination, its dynamic viscosity at room temperature is 65mpa.s.
Embodiment 3
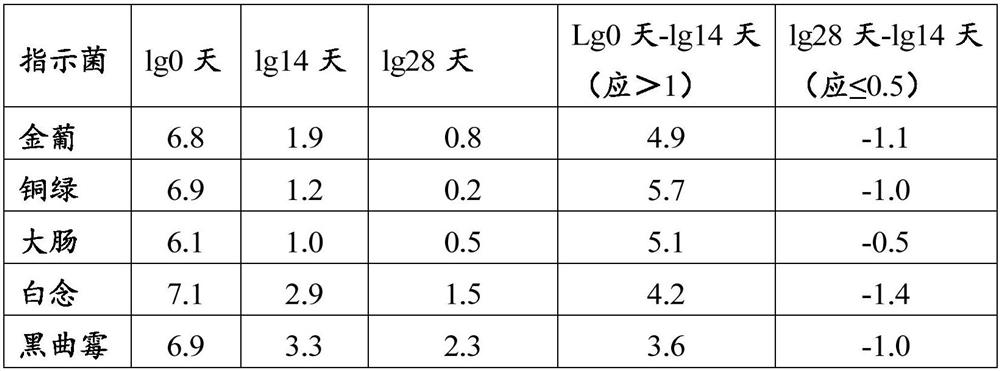
[0044] 1. Weigh 291g of polyethylene glycol 4500, 10.12g of sodium chloride, 1.56g of potassium chloride, 5.06g of sodium bicarbonate, 0.2g of aspartame, appropriate amount of deionized water, and set aside;
[0045] 2. Take an appropriate amount of deionized water, heat it to 50°C, add 10.12g of sodium chloride, 1.56g of potassium chloride, 5.06g of sodium bicarbonate, and 0.2g of aspartame, stir to dissolve completely, and set aside;
[0046] 3. Add polyethylene glycol 4500 291g to the above 2) solution, stir to dissolve completely, add water to 1000ml constant volume, stir to form a uniform and stable solution, put them into 500ml and 25ml containers respectively, stopper and seal Just save it.
[0047] After determination, the dynamic viscosity tested at room temperature is 43mpa.s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com