A method for non-methanol-induced production of antimicrobial peptides
An antibacterial peptide and methanol technology, applied in the fields of bioengineering and biopharmaceuticals, can solve the problems of inapplicability of antibacterial peptide products, unsuitable food or additive production, poor expression effect of antibacterial peptides, etc., and achieve the effect of broad application value and market prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0054] Amplification of Example 1 FLD1 Promoter and Construction of PisL9K22WK Gene Expression Cassette
[0055] According to the AA sequence of the antimicrobial peptide PisL9K22WK, the gene encoding the antimicrobial peptide PisL9K22WK was designed, and the DNA sequence after codon optimization was as follows:
[0056] ATG TTC TTC TTC CAC ATC ATC AAG GGT AAG TTC CAC GCT GGT AGA ATG ATCCAC GGT TTG GTC TGG AAG TAA (SEQ ID NO. 3)
[0057] PCR amplification of FLD1 promoter and PisL9K22WK gene expression cassette
[0058] FLD5BZ (Seq ID NO.4): 5'-GCGAGATCTGCATGCAGGAATCTCTGG-3';
[0059] FLD3HZ (Seq ID NO.5): 5′-GCGGAATTCTGTGAATATCAAGAATTGTATGAACAAGC-3′;
[0060] pis3R (Seq ID NO.6):
[0061] 5'-CCAGCGTGGAATTTGCCTTTGATTATATGAAAGAAGAACATAGGCCTTGTGAATATCAA-3';
[0062] pis3R-pis (Seq ID NO.7): 5'-aatagtGGTACCtcattaTTTCCAGACCAAACCATGAATCATCCTACCAGCGTGGAATTTGCCT-3';
[0063] Using the yeast genome as a template, the reaction system is as follows:
[0064] The PCR reaction syste...
Embodiment 2
[0080] Example 2 Construction of Pichia pastoris intracellular expression vector of antimicrobial peptide PisL9K22WK
[0081] 2.1 The FLDPis-T obtained in Example 1 was double digested with BglII and KpnI endonucleases. At the same time, the pPinkLC vector (purchased from Invitrogen) was double digested with BglII and KpnI.
[0082] The double enzyme digestion system is as follows:
[0083]
[0084] Digestion conditions: 37°C water bath for 1h.
[0085] The digested products were recovered with a gel extraction kit (purchased from TAKARA), and stored at -20°C for future use. Both FLDPis-T and pPinkLC vectors were digested with Bgl II and Kpn I, and ligated with T4 DNA ligase.
[0086] The connection system is as follows:
[0087]
[0088] Ligation conditions: 22°C, 1h; 16°C, 1h; 4°C overnight.
[0089] 2.2 Transform the obtained expression vector into Escherichia coli DH5α: Take out 100 μl of Escherichia coli DH5α competent cells and freeze them at -80°C, place them ...
Embodiment 3
[0101] The construction of embodiment 3PisL9K22WK engineering bacteria
[0102] 3.1 Linearization of expression vector FPLC
[0103] The expression vector FPLC obtained in Example 2 was linearized with SpeI endonuclease and used for PichiaPink yeast transformation.
[0104] The linearization system is as follows:
[0105]
[0106] Reaction conditions: 37°C, 14h.
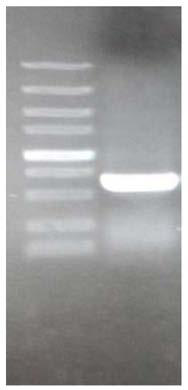
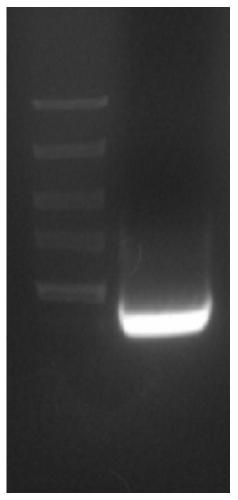
[0107] Figure 4 It is the electrophoresis result of the expression vector FPLC linearized by SpeI digestion in Example 3: among them, M: 1kb DNAmarker, the sample volume is 5 μl; 1: the expression vector FPLC that has not been linearized, the sample volume is 10 μl; 2: linear The expression vector FPLC of the culture, the sample volume is 10 μ l; Electrophoresis result ( Figure 4 ) shows that the expression vector FPLC of the antimicrobial peptide PisL9K22WK is completely linearized.
[0108] 3.2 Preparation of PichiaPink Yeast Competent Cells
[0109] Pick a single colony of PichiaPink yeast (purchased fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com