A multifunctional amphiphilic conjugated molecule material, a preparing method thereof and applications of the material
A conjugated molecule and amphiphilic technology, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems of complex structure, electron injection layer cannot be processed by solution, etc., and achieve low cost, excellent alcohol High solubility and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
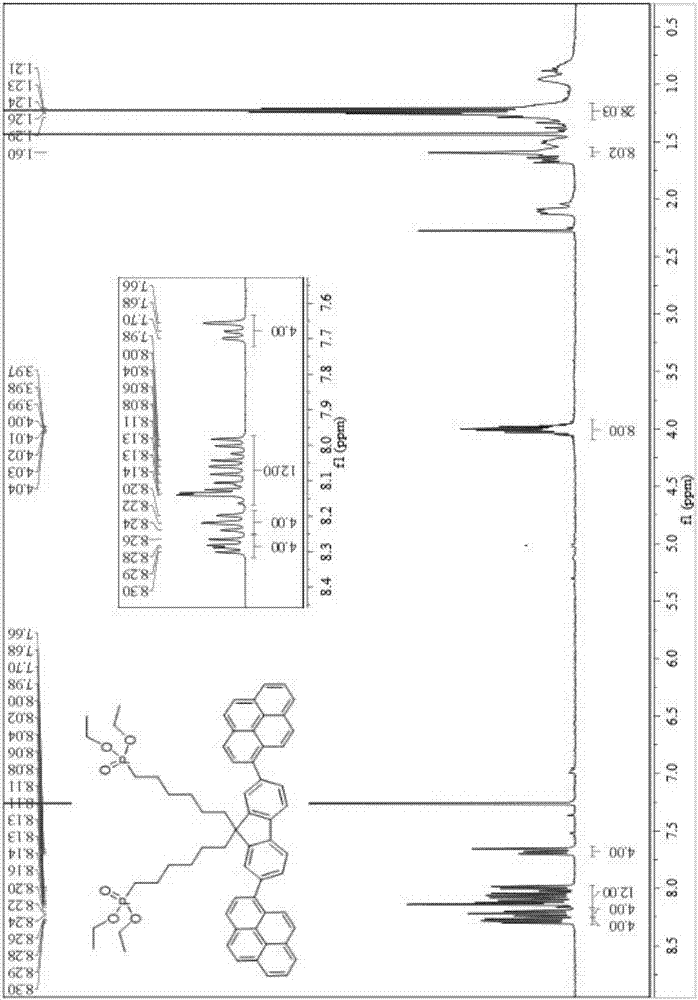
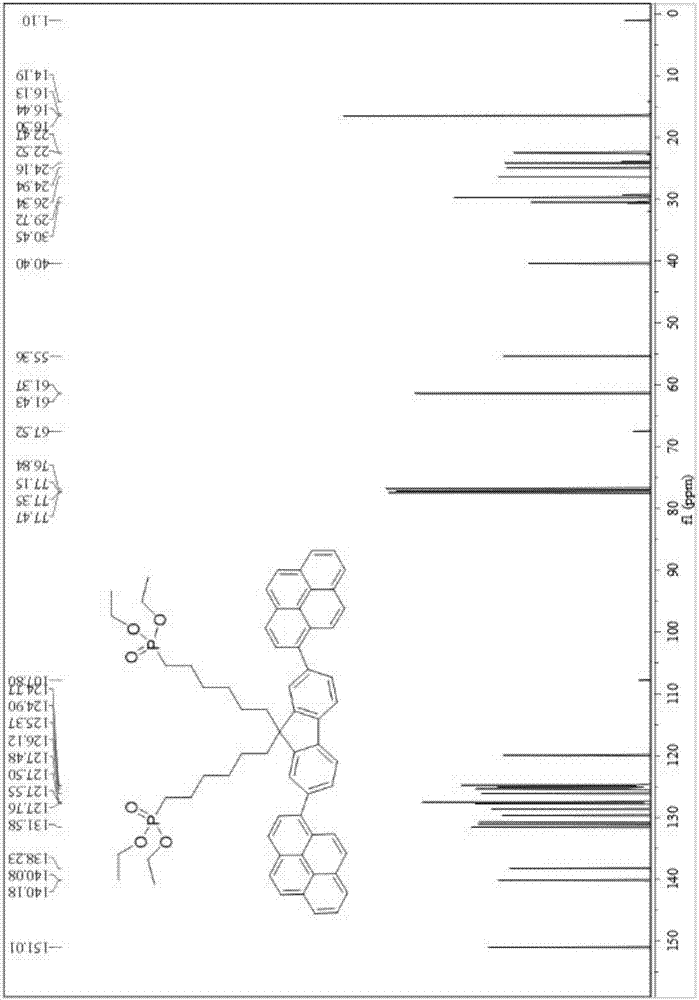
[0048] Embodiment 1: The reaction scheme is as follows.
[0049]
[0050] Specific steps are as follows:
[0051] The first step: 2,7-dibromofluorene (20g, 61.7mmol), 1,6-dibromohexane (35.5mL, 246.9mmol), tetrabutyl ammonium chlorinated ammonium (TBAB) (2g) was added to the quality of 50g Fractions were reacted overnight at 65°C in 50% potassium hydroxide aqueous solution. Extracted, spin-dried, and passed through the column at 100-200 meshes to obtain 32 g of compound A with a yield of 160%.
[0052] Step II: Compound A (6g, 9.23mmol), pinacol borate (9.36g, 36.9mmol) was pumped three times for nitrogen, and then potassium acetate (9.34g, 64.6mmol) was added, nitrogen was pumped, and the catalyst was added in the dark Pd(pddf) 2 Cl 2 (0.4g, 0.524mmol) was dissolved in 45mL of dioxane, and reacted at 100°C for 24h under the protection of nitrogen. Extraction was spin-dried and passed through the column to obtain 4.93 g of compound B with a yield of 82%.
[0053] Step...
Embodiment 2
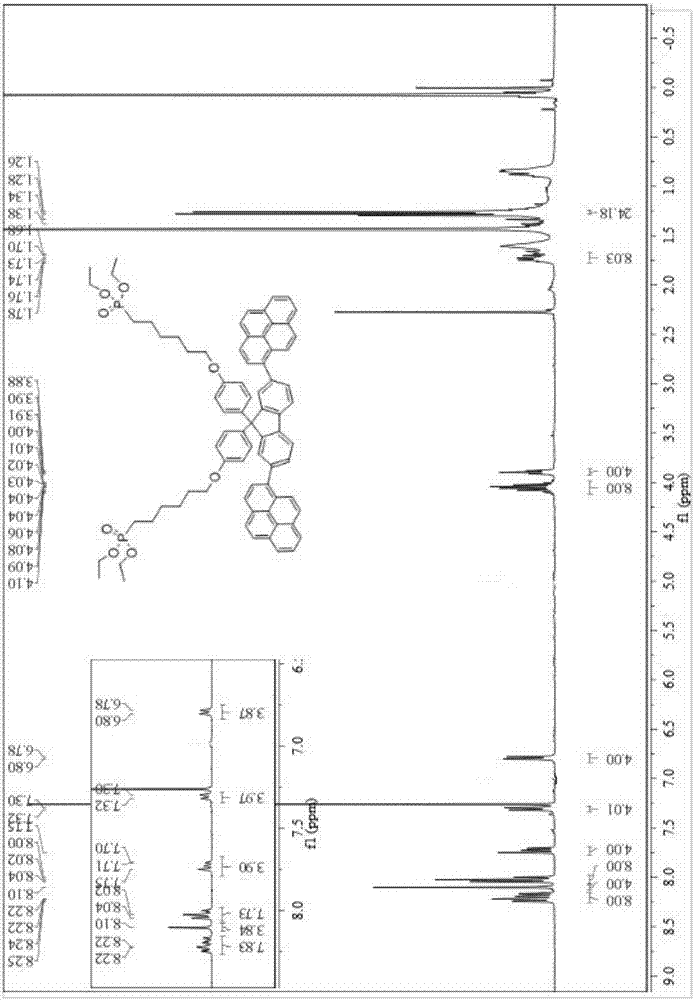
[0057] Embodiment 2: The reaction scheme is as follows.
[0058]
[0059] Specific steps are as follows:
[0060] Step Ⅰ: 2,7-dibromodifluorenone (30.3g, 89.65mmol) was placed in a 500mL three-necked flask, and phenol (71mL, 591.18mmol), 120mL of CCl 4 , the temperature was adjusted to 80°C, and the reaction was carried out for 42h. Stop the reaction, cool with K 2 CO 3 The solution is quenched, and it should be added slowly, and a large number of bubbles will be generated. After suction filtration, the waste liquid is purple. Washed with DCM several times until about 28 g of compound D was pure white in powder form, with a yield of 93%.
[0061] The second step: Compound D (10g, 19.67mmol), 1,6-dibromohexane (19.20g, 78.71mmol), tetrabutyl ammonium olfactory ammonium (2g) adding 50g mass fraction is 50% potassium hydroxide In aqueous solution, react overnight at 65°C. Extract, spin dry, and pass through the column with 100-200 mesh. 13.2 g of compound E was obtaine...
Embodiment 3
[0068] Fabrication of organic light-emitting diodes.
[0069] OLEDs single-layer devices were prepared by spin-coating method: the device structure was ITO / PEDOT:PSS 35nm / luminescent layer 65nm / Al 80nm. Chloroform was used as a solvent, and any one of the compounds PEP / POEP was used for the light-emitting layer to prepare a 15 mg / mL solution. Spin coating under the protection of nitrogen, the rotating speed is 1500rpm, annealing at 80°C for 10min.
[0070] OLEDs single-layer devices were prepared by inkjet printing: the device structure was ITO / PEDOT:PSS 35nm / PEP / Al80nm. Use ethylene glycol methyl ether as a solution to configure 14mg / mL ink. Anneal at 100°C for 20min.
[0071] The following is a comparison of the electroluminescent performance of the above three single-layer organic light-emitting diode devices:
[0072]
[0073] a The turn-on voltage is defined as a brightness of 1cd m -2 when the working voltage. b at maximum brightness. c Prepared by inkjet printi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com