Cyclopeptide compound and preparation method and application thereof
A compound and cyclic peptide technology, applied in the field of cyclic peptide compounds and their preparation and application, can solve the problems of unseen drugs and the like, and achieve the effects of short reproduction cycle, low cost and simple purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
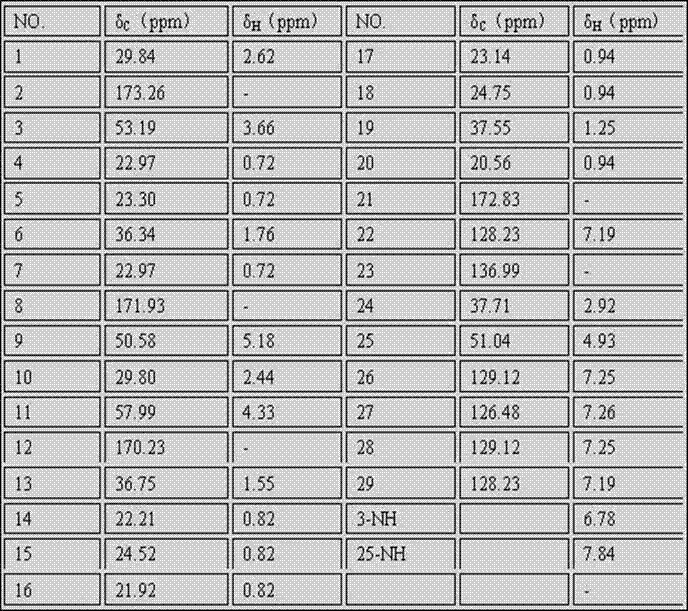
[0025] A cyclic peptide compound, its structural characteristics are: a cyclic tetrapeptide compound composed of 1 leucine residue, 2 N-methyl leucine residues and a phenylalanine residue, the structural formula is shown in I :
[0026] (I).
Embodiment 2
[0028] The preparation method of the cyclic peptide compound shown in I formula specifically comprises the following steps:
[0029] (1) Fermentation production and extraction of extract
[0030] The preservation number is CCTCC No: M2014098 Cladosporium ( Cladosporium sphaerospermum YS3-1-5) colonies were inoculated into 300mL liquid medium (mannitol 10.0g, yeast extract 3.0g, maltose 20.0g, monosodium glutamate 10.0g, glucose 20.0g, KH2PO4 0.5g, MgSO4 0.3g and seawater 1L) In a 1L Erlenmeyer flask (100 flasks in total), culture at 28°C and 180 r / min on a shaker for 10 days to obtain a fermentation broth, which was extracted with ethyl acetate three times, and the combined ethyl acetate extracts were concentrated under reduced pressure and evaporated to dryness , to obtain crude extract (a total of 100 bottles to obtain 20g crude extract);
[0031] (2) Separation and refining
[0032] Dissolve the above crude extract (20g) in a mixed solvent of dichloromethane and methanol...
Embodiment 3
[0038] α-glucosidase inhibitory activity test (96-well plate method)
[0039] (1) Experimental samples
[0040] Preparation of test sample solution: The test sample is the pure compound I isolated and purified in Example 1 above, and an appropriate amount of sample is accurately weighed to prepare a solution with a required concentration for testing the activity.
[0041] (2) Experimental method
[0042] 4-Nitrophenyl-α-D-glucopyranoside was used as the substrate, reacted on a 96-well microtiter plate, and the final reaction volume was 200 μL, and the α-glucosidase inhibitory activity was determined. Add different concentrations of sample solutions (40 μL) to 40 μL 0.04 U / mL α-glucosidase solution, react at 37°C for 5 minutes, add 20 μL 0.5 mmol / L 4-nitrophenyl-α-D-glucopyranose Glycoside solution, after reacting at 37°C for 30min, add 100μL 0.1mol / L Na 2 CO 3 The solution terminates the reaction, and the absorbance of the solution is measured at a wavelength of 405nm. Ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com