Organic solar cell with cyclopentyl bithiophene derivative as electron acceptor
A technology of organic solar cells and electron acceptors, applied in the field of solar cells, can solve problems such as deterioration of photovoltaic performance and decline in exciton separation efficiency, and achieve the effects of low cost, outstanding photovoltaic performance, and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Using simple and easy-to-obtain cyclopentadithiophene as the initial chemical raw material, by introducing an alkyl side chain, connecting an organotin group on one side, coupling with a difluorophenyl group, connecting an aldehyde group on both sides, and finally connecting with an IC The end groups are connected, and a total of five steps of chemical reactions are used to obtain the target product DFPCIC. The specific synthetic route is as follows:
[0019]
[0020] The specific synthesis steps of DFPCIC are:
[0021] (1) Synthesis of Intermediate 2
[0022] 1.78 g cyclopentadithiophene, 4 g bromoethylhexyl, 1.84 g KOH and 0.24 g KI were dissolved in 40 mL dimethyl sulfoxide (DMSO), and stirred overnight at room temperature. The reaction solution was extracted with ether, the organic phase was collected, washed 3 times with water, and the solvent was removed by rotary evaporation. The crude product was purified with a silica gel column using n-hexane as the eluent...
Embodiment 2
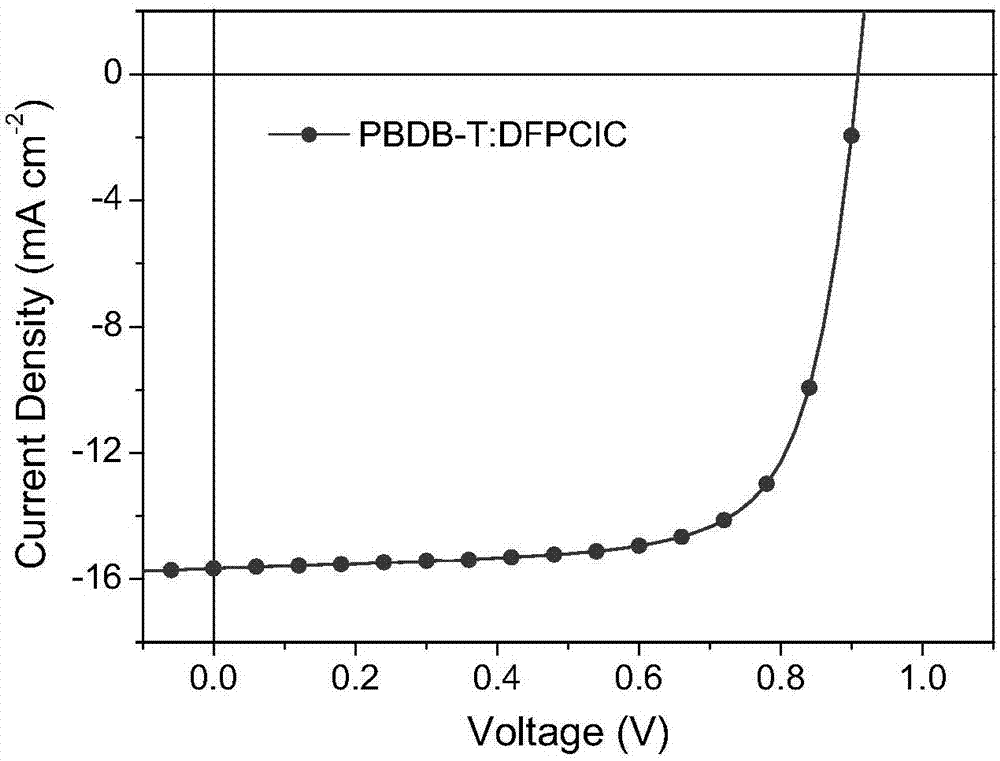
[0033]The transparent conductive glass with strip-shaped ITO (cathode) etched on the surface is cleaned with cleaning agent, deionized water, acetone and isopropanol by ultrasonic oscillation, dried, and then treated with ultraviolet ozone for 15 minutes; A layer of ZnO was spin-coated at 3500 rpm for 60 seconds, and then annealed at 170° C. for 20 minutes. Then the sheet was transferred to the glove box, and a layer of PFN was spin-coated on the ZnO with a PFN solution of 0.4 mg / mL, the rotating speed was 3000 rpm, and the spin-coating time was 60 seconds. Afterwards, the PBDB-T:DFPCIC mixture with a weight ratio of 1.5:1 and a total concentration of 20 mg / mL was spin-coated at a speed of 2000 rpm for 60 seconds to obtain an active layer with a thickness of 100 nm. Finally, a layer of MoO with a thickness of 10 nm was evaporated with an evaporation apparatus. 3 interface layer and a 100nm thick Ag electrode (anode), resulting in an active area of 6 mm 2 organic solar cell...
Embodiment 3
[0036] The transparent conductive glass with strip-shaped ITO (cathode) etched on the surface is cleaned with cleaning agent, deionized water, acetone and isopropanol by ultrasonic oscillation, dried, and then treated with ultraviolet ozone for 15 minutes; A layer of ZnO was spin-coated at 3500 rpm for 60 seconds, and then annealed at 170° C. for 20 minutes. Then the sheet was transferred to the glove box, and a layer of PFN was spin-coated on the ZnO with a PFN solution of 0.4 mg / mL, the rotating speed was 3000 rpm, and the spin-coating time was 60 seconds. Afterwards, the PBDB-T:DFPCIC mixture with a weight ratio of 1:1 and a total concentration of 20 mg / mL was spin-coated at a speed of 2000 rpm for 60 seconds to obtain a 100 nm-thick active layer. Finally, a layer of MoO with a thickness of 10 nm was evaporated with an evaporation apparatus. 3 interface layer and a 100nm thick Ag electrode (anode), resulting in an active area of 6 mm 2 organic solar cells.
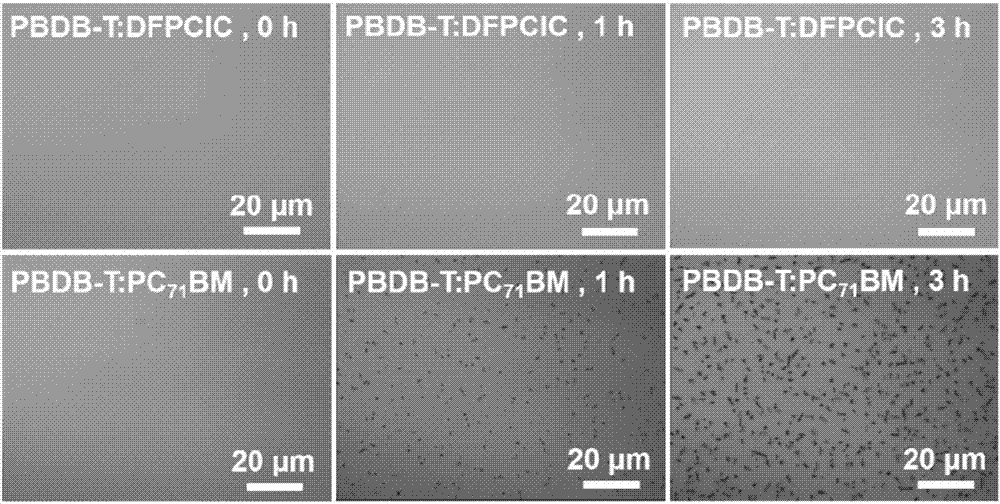
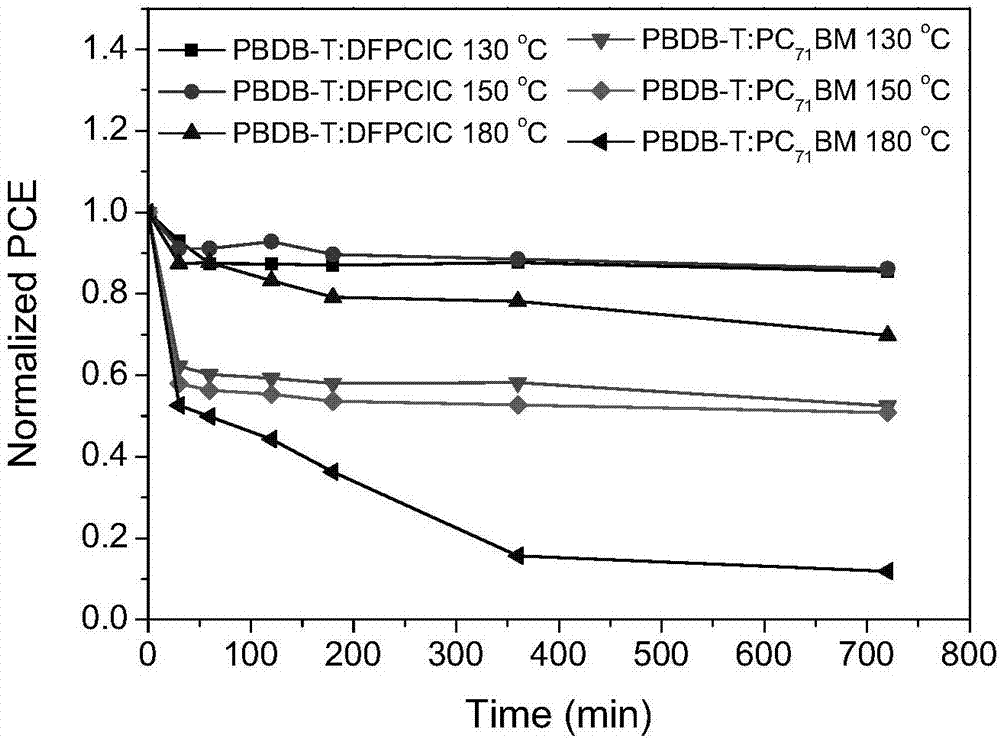
[0037] Whe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com