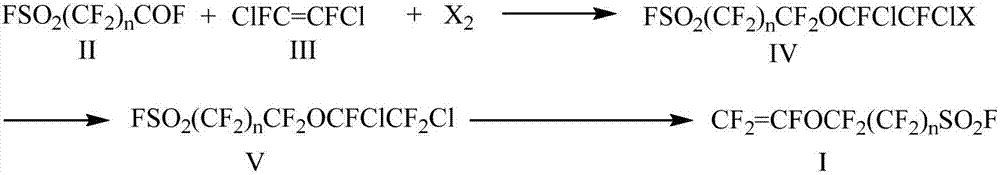Preparation method for perfluoro vinyl ether with sulfuryl fluoride group as terminal group
A technology of perfluorovinyl ether and fluorosulfonyl perfluoroalkyl acyl, applied in the field of fine chemicals, can solve the problem of complex synthesis and preparation methods, low yield, high cost of short-chain sulfonyl fluoride monomers, and inability to generate in one step problems such as alkenyl ether double bonds, to achieve the effects of low cost, avoidance of waste and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] FSO 2 CF 2 CF 2 o - K + Preparation of:
[0044] 1 liter three-necked flask, equipped with a thermometer and agitator, add 200mL of diethyl ether into the three-necked flask, then add 60g of potassium fluoride, then add FSO dropwise 2 CF 2 COF liquid (180g), the water bath controls the temperature in the reaction bottle to not exceed 25°C. After the addition, continue to stir at room temperature for 24 hours, and check that there is no residual acyl fluoride reagent FSO in the reaction solution 2 CF 2 After COF, the reaction mixture was transferred to a single-necked flask and spin-dried to obtain 235 g of yellow powder. Characterized correctly by infrared and NMR, in 19 In the FNMR spectrum, it was found that there was -28ppm of CF 2 OK structure.
[0045] FSO 2 CF 2 CF 2 Preparation of OCFClCFICl(IVa):
[0046] Under anhydrous and oxygen-free conditions, add 800 mL of diglyme to a 2L three-necked flask, and then add the prepared potassium alkoxide FSO un...
Embodiment 2
[0052] The preparation method is the same as Example 1, the only difference is: FSO 2 CF 2 CF 2 OCFClCF 2 The preparation of Cl(V) adopts the following method:
[0053] In a 500mL three-necked flask equipped with a reflux condenser, a stirrer and a constant pressure dropping funnel, add 60g of dry antimony trifluoride and 20mL of antimony pentachloride. When the internal temperature of the reaction bottle reaches 140°C, slowly add FSO 2 CF 2 CF 2 OCFClCFICl(IV) 100g, continue to reflux for 2-5h after the dropwise addition, and then fractionally distill to obtain a fraction at 117-120°C with a yield of 83%.
Embodiment 3
[0055] The preparation method is the same as Example 1, the only difference is: FSO 2 CF 2 CF 2 The preparation of OCFClCFBrCl (IVb) adopts the following method:
[0056] Under anhydrous and oxygen-free conditions, add 800 mL of diglyme to a 2L three-necked flask, and then add the prepared potassium alkoxide FSO under stirring. 2 CF 2 CF 2 o - K + , and then add 165g of liquid bromine, under ice bath (control the internal temperature of the reaction bottle not to exceed 10°C), slowly add difluorodichloroethylene (140g) solution dropwise, the dropwise addition is completed in 4 to 8 hours, and after continuing to react for 4 hours, the reaction is mixed Pour the liquid into ice water, and add 10% sodium thiosulfate aqueous solution to wash and extract the layers, and then rectify under reduced pressure to obtain the product FSO 2 CF 2 CF 2 OCFClCFBrCl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com