Preparation method of zinc stannate nanocube or nanosheet material
A nano-cube, zinc stannate technology, applied in chemical instruments and methods, tin compounds, inorganic chemistry, etc., can solve the problem of high energy consumption, achieve the effects of good reproducibility, easy mass production and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
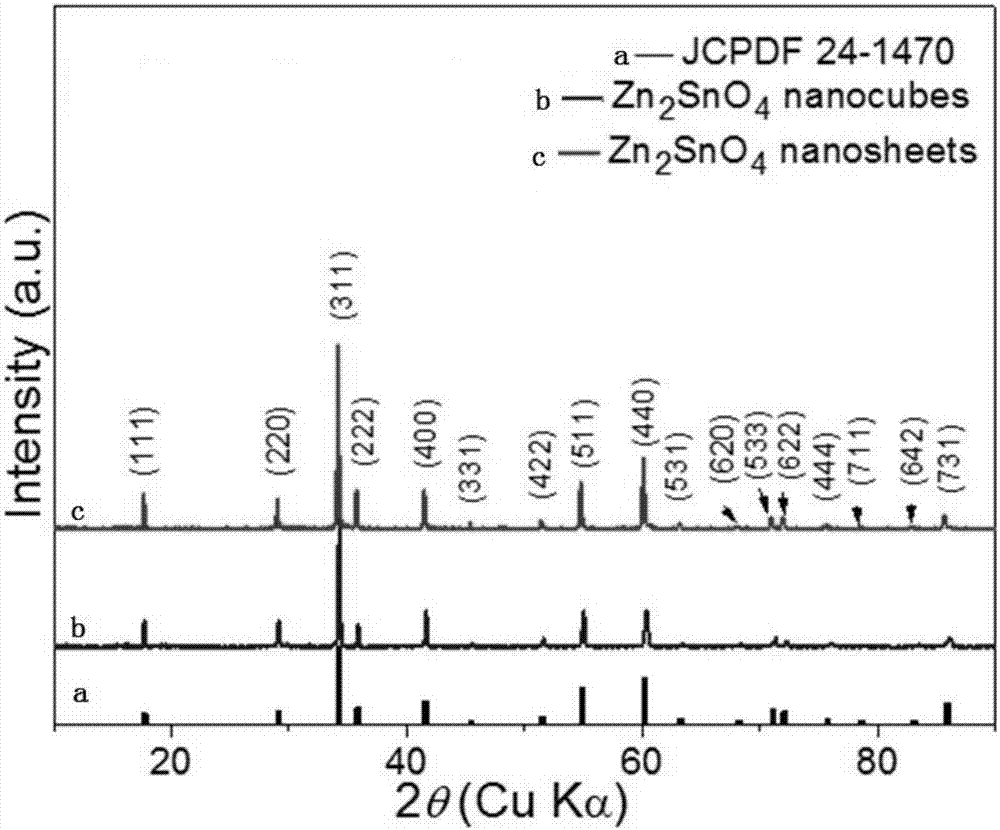
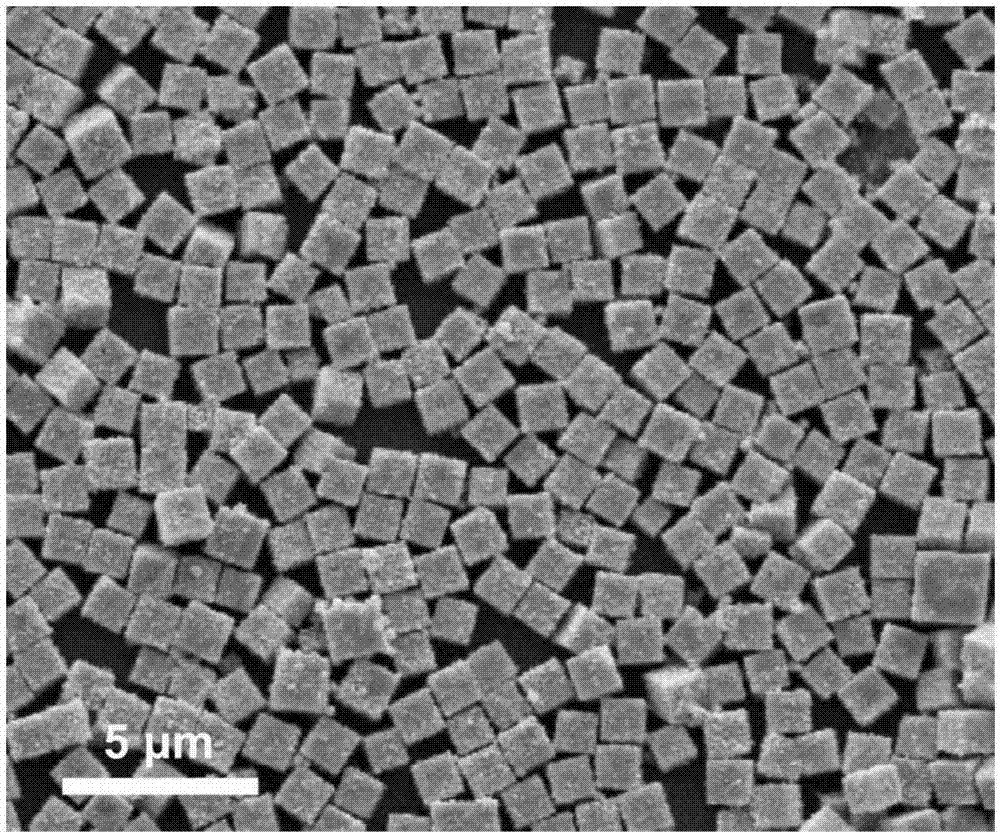
[0030] Add 1mmoL zinc acetate, 0.5mmoL tin tetrachloride and 1mmoL sodium dodecylbenzenesulfonate into a 50mL Erlenmeyer flask containing a mixture of 15mL water and 15mL ethanol, and stir in a water bath at 30°C for 120min. Then, 3 mL of tetraethylamine hydroxide was added dropwise to the above solution, reacted for 90 min, and set aside. Pour the above-mentioned zinc stannate precursor solution into a 50mL polytetrafluoroethylene-backed reaction kettle, seal it, place it in an oven, control the temperature of the oven at 180°C, react for 5 hours, cool to room temperature, wash and vacuum Dry to obtain zinc stannate cubes. figure 1 The positions and relative intensities of the diffraction peaks are consistent with the JPCDS card (24-1470) of the zinc stannate cube, indicating that the product zinc stannate has a high degree of crystallinity. from figure 2 It can be seen that the prepared zinc stannate cubes are uniformly dispersed and regular in shape, and the average edge...
Embodiment 2
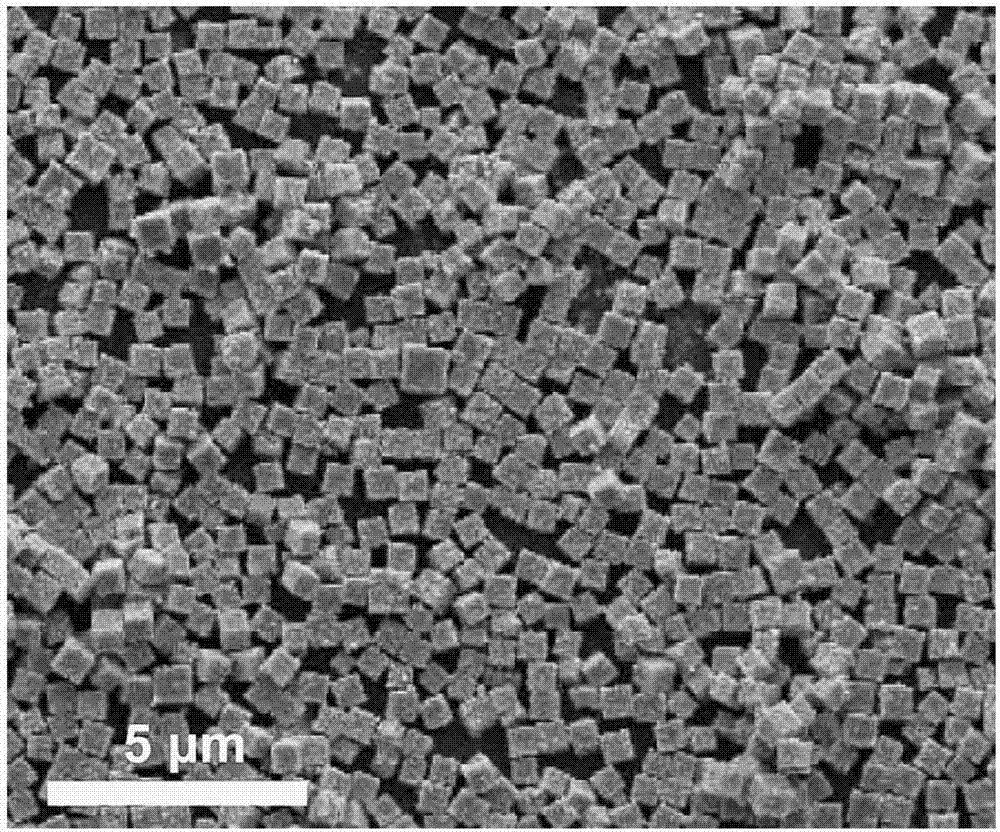
[0032] Add 1mmoL of zinc nitrate, 0.5mmoL of tin tetrachloride and 1mmoL of sodium dodecylbenzenesulfonate into a 50mL Erlenmeyer flask containing a mixture of 15mL of water and 15mL of ethanol, and stir in a water bath at 60°C for 30min. Then, 3 mL of tetraethylamine hydroxide was added dropwise to the above solution, reacted for 1 h, and set aside. Pour the above-mentioned zinc stannate precursor solution into a 50mL polytetrafluoroethylene-backed reaction kettle, seal it, place it in an oven, control the temperature of the oven at 220°C, react for 8 hours, cool to room temperature, wash it several times, vacuum After drying, large-sized zinc stannate cubes are obtained. figure 1 The positions and relative intensities of the diffraction peaks are consistent with the JPCDS card (24-1470) of the zinc stannate cube, indicating that the product zinc stannate has a high degree of crystallinity. from image 3 It can be seen that with the prolongation of the reaction time, the cu...
Embodiment 3
[0034] Add 1mmoL of zinc chloride, 0.5mmoL of tin tetrachloride and 0mmoL of sodium dodecylbenzenesulfonate into a 50mL Erlenmeyer flask containing a mixture of 15mL of water and 15mL of ethanol, and stir in a water bath at 60°C for 30min. Then, 3 mL of tetraethylamine hydroxide was added dropwise to the above solution, reacted for 1 h, and set aside. Pour the above-mentioned zinc stannate precursor solution into a 50mL polytetrafluoroethylene-backed reaction kettle, seal it, place it in an oven, control the temperature of the oven at 220°C, react for 8 hours, cool to room temperature, wash it several times, vacuum After drying, zinc stannate nanosheets are obtained.
[0035] figure 1 The positions and relative intensities of the diffraction peaks are consistent with the JPCDS card (24-1470) of zinc stannate nanosheets, indicating that the product zinc stannate has a high degree of crystallinity. from Figure 4 It can be seen that when other reaction conditions remain uncha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com