Method for preparing ethyl methyl carbonate from dimethyl carbonate and diethyl carbonate
A technology of diethyl carbonate and dimethyl carbonate, applied in the field of preparation of chemical raw materials, which can solve problems such as separation and recovery difficulties, limited application, and difficult to meet raw material requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
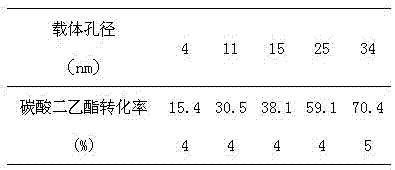
[0071] In the fixed-bed reactor, self-made and single macroporous SiO 2 As a carrier, Ca(NO 3 ) 2 and Mg(SO 4 )2 25%Ca-5%Mg / SiO solution 2 Catalyst (1#), with single small hole SiO 2 As a carrier, Ca(NO 3 ) 2 and Mg(SO 4 ) 2 25%Ca-5%Mg / SiO solution 2 Catalyst (2#) and SiO composited with large and small pores 2 -Al 2 o 3 As a carrier, Ca(NO 3 ) 2 and Mg(SO 4 ) 2 25%Ca-5%Mg / SiO solution 2 Catalyst (3#) 50 g each, coprecipitation agents are KOH and NaHCO 3 dimethyl carbonate and ethanol were pumped into the reactor with a molar ratio of 1:1 using a constant-flow pump, and the reaction temperature was At 150°C, the mass space velocity of diethyl carbonate is 30h -1 After a stable reaction for 500 h under certain reaction conditions, samples were taken for chromatographic analysis and calculation, and the results are shown in Table 1.
[0072]
[0073] According to the reaction equation, dimethyl carbonate and diethyl carbonate react at an approximate molar r...
Embodiment 2
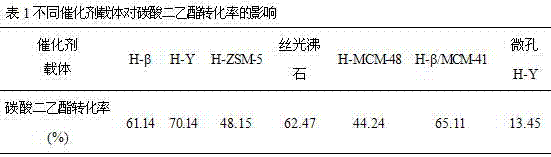
[0076] In the fixed bed reactor, the CaCl 2 and MgCl 2 25%Ca-5%Mg / SiO solution 2 Catalyst (4#), use Ca(NO 3 ) 2 and Mg(NO 3 ) 2 25%Ca-5%Mg / SiO solution 2 Catalyst (5#), use Ca(SO 4 ) 2 and Mg(SO 4 ) 2 25%Ca-5%Mg / SiO solution 2 Catalyst (6#), use CaCl during coprecipitation 2 and Mg(NO 3 ) 2 25%Ca-5%Mg / SiO solution 2 Catalyst (7#) and co-precipitation using Ca(NO 3 ) 2 and Mg(SO 4 ) 2 25%Ca-5%Mg / SiO solution 2 Catalyst (3#) 50 g each, coprecipitation agents are KOH and NaHCO 3 dimethyl carbonate and ethanol were pumped into the reactor with a molar ratio of 1:1 using a constant-flow pump, and the reaction temperature was At 150°C, the mass space velocity of diethyl carbonate is 30h -1 After 500 h of stable reaction under the reaction conditions, samples were taken for chromatographic analysis and calculation, and the results are shown in Table 2.
[0077]
[0078] As can be seen from Table 2, the use of Ca(NO 3 ) 2 and Mg(SO 4 ) 2 The mixed salt sol...
Embodiment 3
[0080] In the fixed-bed reaction tube, the co-precipitation prepared with different co-precipitation agents were loaded respectively, and Ca(NO 3 ) 2 and Mg(SO 4 ) 2 A mixed salt solution of 25%Ca-5%Mg / SiO as the source of active components 2 Catalyst 50 g, marked as NaOH (8#), KOH (9#), NH 3 ·H 2 O (10#), KOH and NaHCO 3 The mixed alkali (3#), NaOH and NH 3 ·H 2 Mixed base of O (11#), KOH and NH 3 ·H 2 The mixed alkali (12#) co-precipitation temperature of O was 70 °C, the co-precipitation pH was 11, and the aging time was 20 h. Use a constant flow pump to pump dimethyl carbonate and diethyl carbonate into the reactor at a molar ratio of 1:1. The reaction temperature is 150°C and the mass space velocity of diethyl carbonate is 30h. -1 After stable reaction for 500 h under the reaction conditions, samples were taken for chromatographic analysis and calculation, and the results are shown in Table 3.
[0081]
[0082] It can be seen from Table 3 that different co-p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| mesopore | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com