Cytomembrane targeting H2S fluorescence probe as well as preparation method and application thereof
A fluorescent probe, H2S technology, used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effects of efficient and sensitive analysis and detection, simple and easy preparation methods, and significant changes in fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
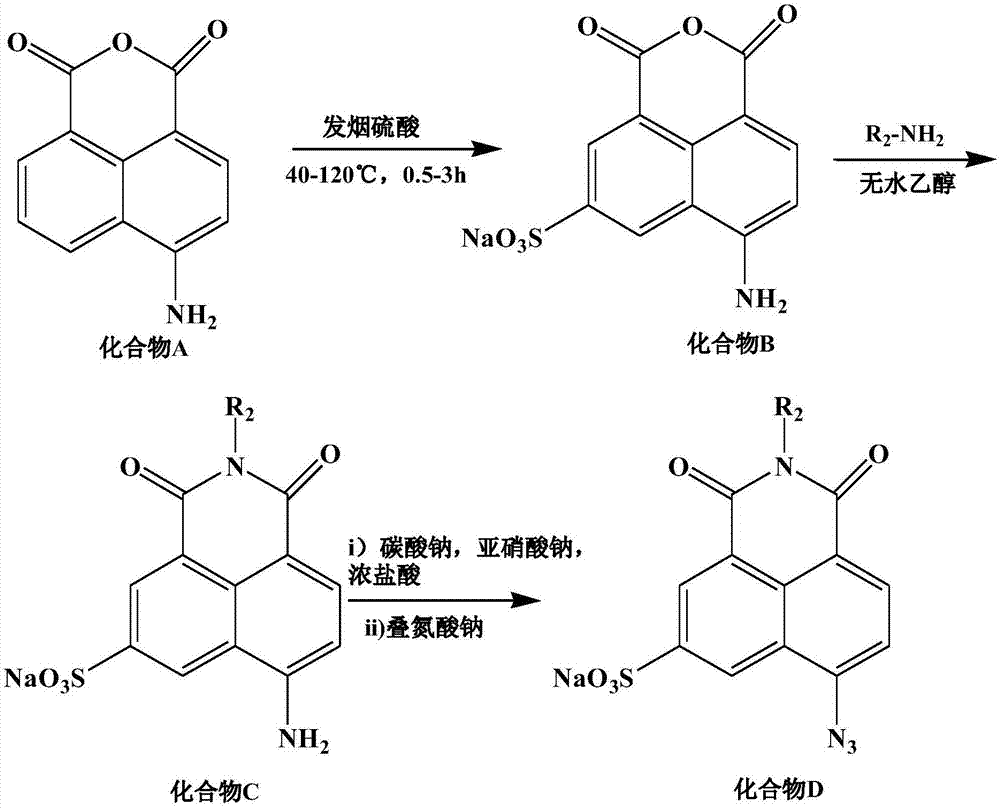
[0029] Intermediate 4-amino-6-sulfonic acid-1,8-naphthalene dicarboxylic anhydride ( figure 1 Synthesis of compound B) in:
[0030] To 0.204g 4-amino-1,8-naphthalene dicarboxylic anhydride ( figure 1 Add 4mL of 50% fuming sulfuric acid to the compound A), stir and react at 100°C for 1 hour, after cooling to room temperature, add 50mL of ice water, then add 7g of sodium chloride for stirring, and filter after forming a suspension The precipitate was collected, washed successively with 2 mL of water, 30 mL of ethanol, and 30 mL of ether, and dried in vacuo to obtain 0.235 g of orange-red powder solid Compound B with a yield of 74%. The intermediate was soluble in water, methanol, and dimethyl sulfoxide; insoluble in ethanol.
Embodiment 2
[0032] Intermediate 4-amino-6-sulfonic acid-N-hexadecyl-1,8-naphthalimide ( figure 1 Synthesis of compound C) in:
[0033] (1) The good solvent of compound B is preferred as the reaction medium. Dissolve 0.300g of Compound B in 15mL of water, add 10 drops of triethanolamine dropwise, then add 10mL of water suspension dispersed with 0.724g of hexadecylamine, and react in an oil bath at 100°C for 18 hours. After the reaction is completed, cool to room temperature. Compound C is obtained.
[0034] (2) Methanol is a good solvent for compound B and raw material hexadecylamine. Dissolve 0.300g of compound B in 15mL of methanol, add 10 drops of triethanolamine dropwise, then add 10mL of methanol solution containing 0.724g of hexadecylamine, react in an oil bath at 90°C for 18 hours, cool to room temperature after the reaction, and collect the precipitate by filtration , washed with anhydrous ether, the resulting precipitate was characterized as an impurity, not compound C.
[003...
Embodiment 3
[0037] Target product 3-sulfonic acid (sodium)-5-azido-N-hexadecyl-1,8-naphthalimide ( figure 1 Compound D) in the synthesis:
[0038] Dissolve 150mg of Compound C in 15mL of water, place it in an ice-salt bath after dissolving, add dropwise 1mL of an aqueous solution containing 154mg of sodium nitrite, add 3mL of concentrated hydrochloric acid within 15 seconds after completion, stop stirring, and place it in an ice-salt bath for 4 After the reaction was completed, the brown precipitate was collected by filtration and washed with 1 mL of ice water. The collected solid was dispersed in anhydrous methanol at -50°C to form a suspension, and a methanol solution containing 181 mg of sodium azide in 15 mL was added within 10 minutes. The reaction was stirred at -50°C for 6 hours, filtered with a G4 sand core funnel, the filtrate was evaporated to dryness at room temperature, and the resulting solid was purified by silica gel column chromatography, and the eluent was dichloromethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com