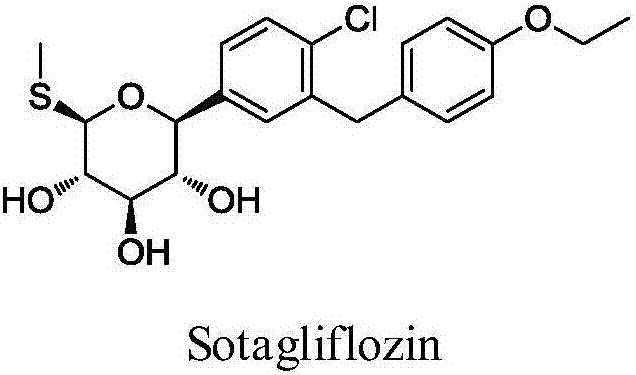
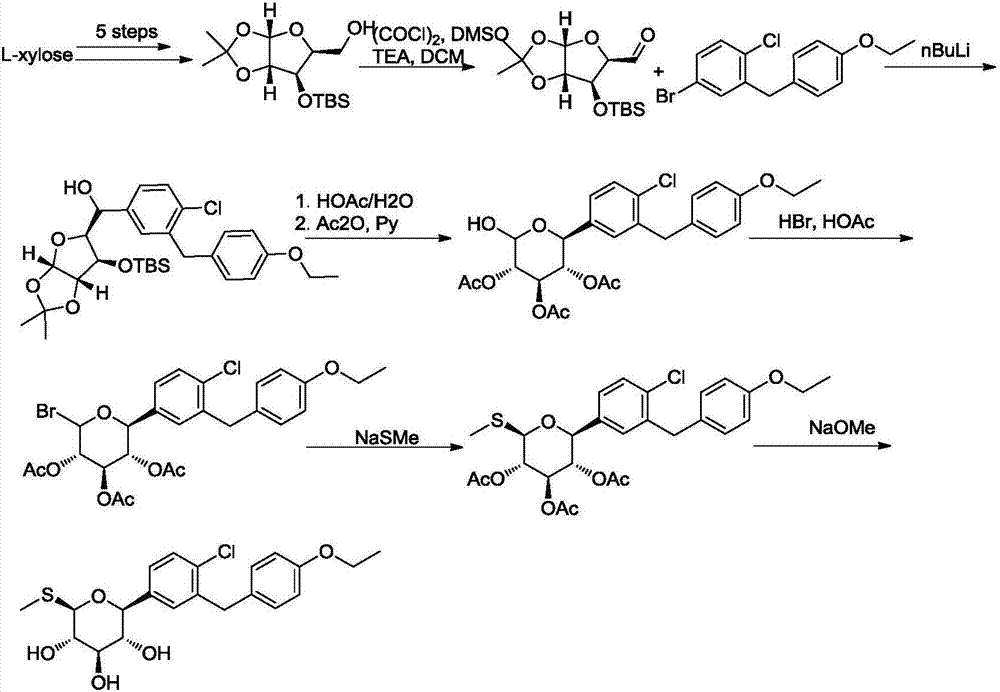
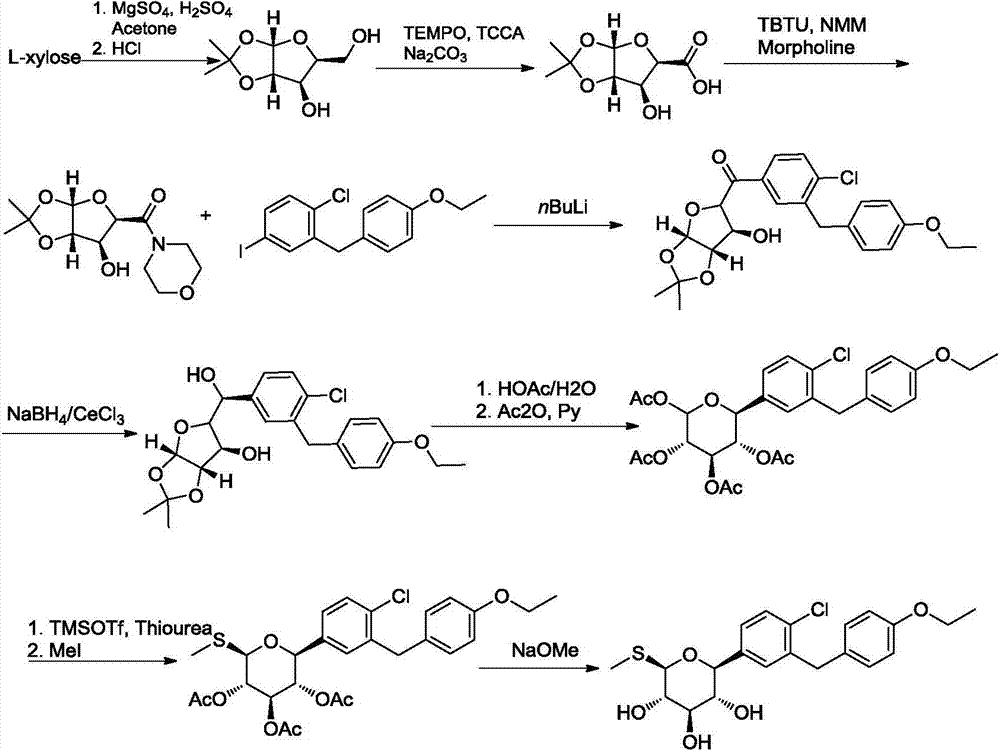
Sotagliflozin preparing method and an intermediate thereof
An intermediate and synthetic method technology, applied in the field of preparation of Sotagliflozin, can solve the problems of low efficiency, low reaction yield, high requirements for reaction equipment and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075]
[0076] Compound formula 1A (19.02g, 100mmo) was dissolved by adding dichloromethane (190mL), adding diisopropylethylamine (25.95g, 200mmol), DMAP (1.22g, 10mmol), stirring evenly and cooling to 0-5°C. TBSCl (16.58 g, 110 mmol) was added dropwise, and after the drop was completed, the temperature was raised to room temperature to react overnight. After the reaction was completed, saturated ammonium chloride (190 mL) was added, the aqueous phase was extracted once with dichloromethane (190 mL), the combined organic phase was washed twice with saturated brine (190 mL), and the solvent was spin-dried to obtain the crude compound 1B, which was directly used in the next reaction.
[0077] The base diisopropylethylamine here can be replaced by pyridine, triethylamine, DBU or DABCO, the catalyst DMAP can be used without adding, and the solvent dichloromethane can be 1,2-dichloroethane, N,N-dimethylformamide , N,N-dimethylacetamide, N-methylpyrrolidone, acetonitrile, tetrah...
Embodiment 2
[0079]
[0080] Add compound 1B (100mmol, obtained from Example 1) and tetrahydrofuran (152mL) into a three-necked flask, stir evenly, cool to 0-5°C, add lithium tert-butoxide (13.47g, 120mmol), and stir at low temperature for 20-30 minutes Benzyl bromide (18.81 g, 110 mmol) was added dropwise, and after the addition was completed, it was raised to room temperature at 25-30° C. for 6-8 hours. After the reaction, add saturated ammonium chloride (152mL), extract with ethyl acetate (152mL) for 3 times, combine the organic phases and wash with saturated brine once (152mL), dry over anhydrous sodium sulfate, and concentrate the oily 1Ca crude product directly into the next reaction .
[0081] Lithium tert-butoxide can be replaced here by potassium carbonate, sodium carbonate, cesium carbonate, sodium hydroxide, potassium hydroxide, sodium hydride, potassium tert-butoxide or sodium tert-butoxide.
Embodiment 3
[0083]
[0084] Compound 1Ca (100mmol, obtained in Example 2) and tetrahydrofuran (395mL) were added into a three-neck flask, stirred evenly, and TBAF (94.65g, 300mmol) was added. After the addition, the mixture was raised to room temperature at 25-30°C for 6-8 hours. After the reaction, add saturated ammonium chloride (152mL), extract with ethyl acetate (152mL) for 3 times, combine the organic phases and wash with saturated brine once (152mL), dry over anhydrous sodium sulfate, concentrate the oily 1Da crude product and put it directly into the next reaction .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com