Synthesis method of (R)-2-(1-aminoethyl)-4-fluoroaniline
A synthesis method and aminoethyl technology, which are applied in the preparation of aminohydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of difficult reaction conditions, low yield, long steps, etc., and achieve low reaction temperature, avoidance of Effects of pollution, mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
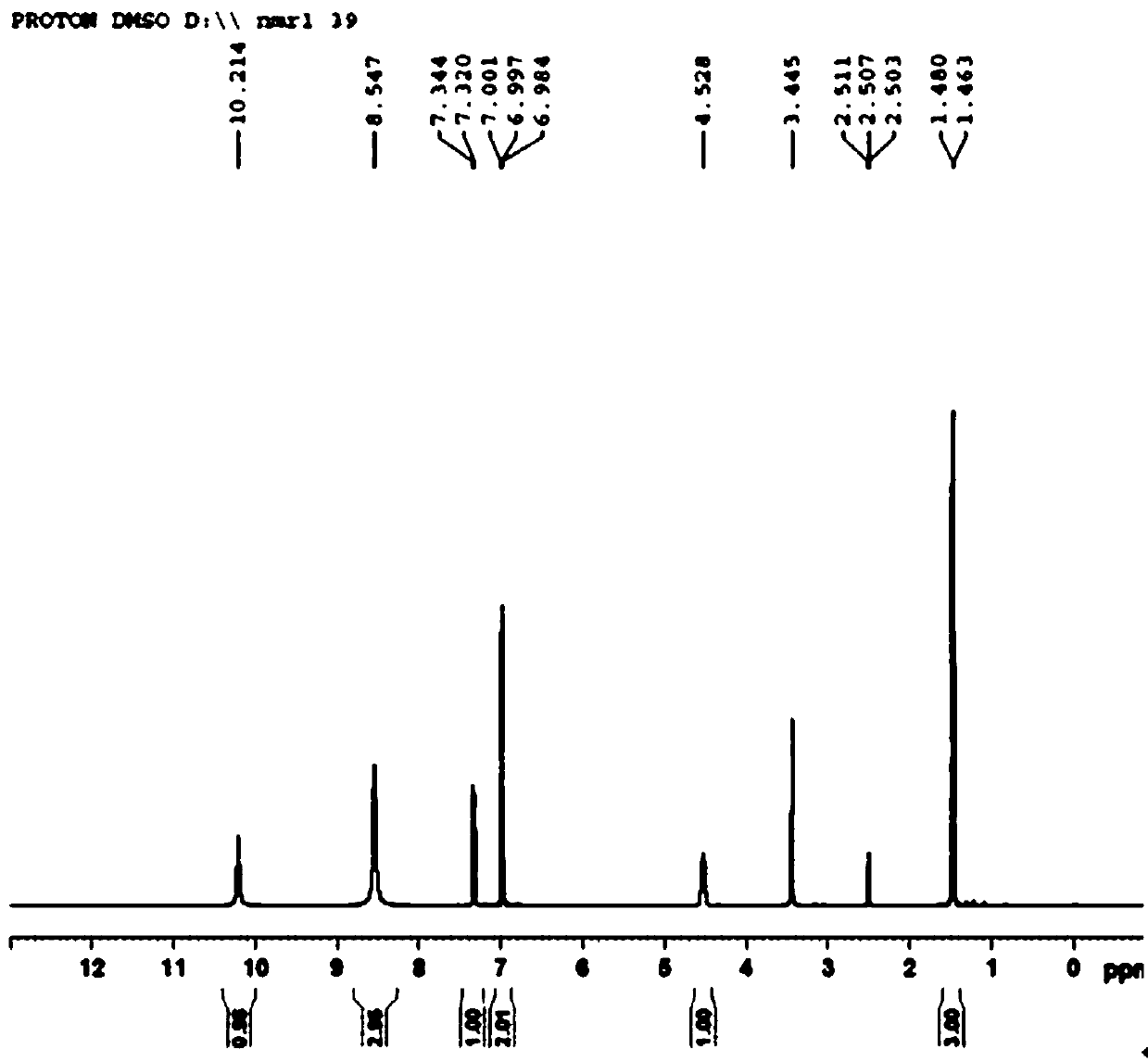
[0037] The invention provides a kind of synthetic method of (R)-2-(1-aminoethyl)-4-fluoroaniline, comprising the following steps:
[0038] S1. Solution preparation of raw materials, the coenzyme is prepared into a coenzyme aqueous solution, and the amino donor is prepared into an amino donor aqueous solution; the amino donor is isopropylamine, sec-butylamine or DL-alanine. The coenzyme is pyridoxal phosphate, and the coenzyme concentration ranges from 10.0 mmol / L to 20.0 mmol / L.
[0039] S2. Add the solution prepared in S1 to the reaction bottle added with the buffer and stir to mix. The pH of the buffer is 7-9; the buffer is potassium phosphate aqueous solution or Tris buffer .
[0040] S3. Add transaminase dry powder to the mixed solution of S2, stir evenly and then add the co-solvent with the substrate dissolved. The weight ratio of the transaminase dry powder to the substrate is 0.5%~1%, and the vacuum degree of the system is kept at -0.03 Mpa~-0.06Mpa, heating to 25~35°...
Embodiment 1
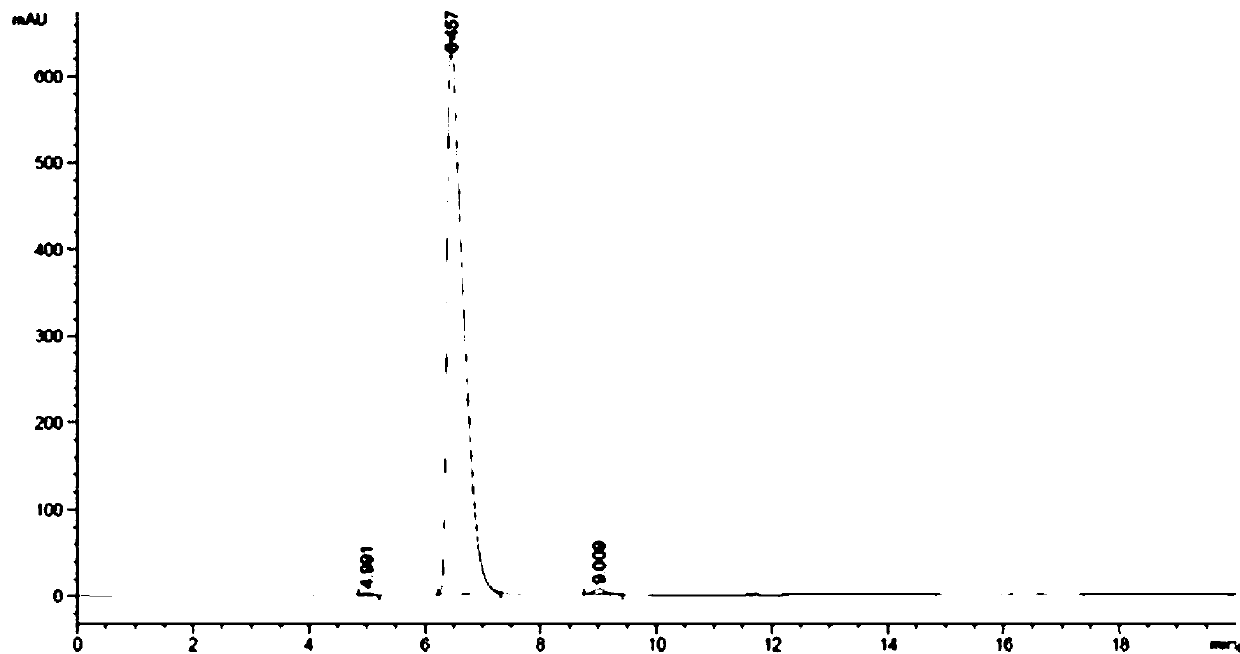
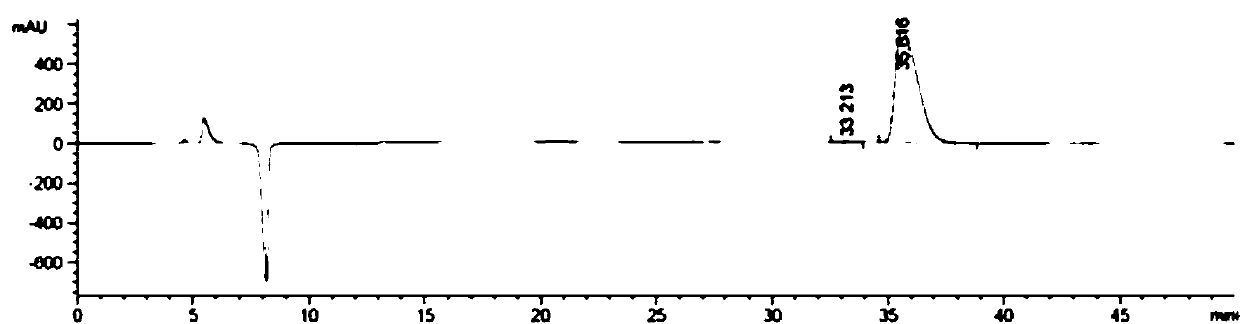
[0050] Add 150 mL of dipotassium hydrogen phosphate buffer solution (pH 8) to a 2 L reaction bottle, add 150 mL of the pre-prepared 10mmol / L coenzyme pyridoxal phosphate (PLP) aqueous solution, add the pre-prepared 1M / L 1.29L of isopropylamine aqueous solution (the pH of the aqueous solution was adjusted to 8 with 10% phosphoric acid), and the stirring was started slowly. Then add 15 mg of transaminase dry powder Cat.No.1.2.126, stir for 5 minutes and then add dimethyl sulfoxide solution of 2-hydroxy-5-fluoroacetophenone (dissolve 10 g of raw material ketone in 75 mL of dimethyl sulfoxide ). Turn on the vacuum to system -0.03MPa. Turn on the heat to 35°C. After 18 hours of reaction, the liquid phase showed that <1.0% of the raw material remained. Stop stirring, filter with suction, and rinse the filter cake with 50 mL of isopropyl acetate. The filtrate was transferred to a 5 L reaction flask, and 1M / L dilute hydrochloric acid was added under stirring to adjust the pH of th...
Embodiment 2
[0053] Add 150mL Tris-HCl buffer solution (pH 8), pre-prepared 10 mmol / L coenzyme pyridoxal phosphate (PLP) aqueous solution 150mL, pre-prepared 1.0M / L secondary 1.29L of butylamine aqueous solution (adjust the pH to 8 with 10% phosphoric acid), slowly start stirring; then add 50mg of transaminase dry powder of Cat.No. 1.2.132, stir for 5 minutes, then add 2-hydroxy-5-fluoro Dimethyl sulfoxide solution of acetophenone (10 g of raw ketone dissolved in 100 mL of dimethyl sulfoxide). Turn on the vacuum to the system -0.05MPa. Turn on the heat to 35°C. After 18 h of reaction, the liquid phase showed that <1.0% of the starting material remained. Stop stirring, and filter with suction; the filter cake is rinsed with 50 mL of isopropyl acetate. The filtrate was transferred to a 5L reaction flask, and 1M / L dilute hydrochloric acid was added under stirring to adjust the pH of the system to 3-4. Then add 200 mL of isopropyl acetate to extract the aqueous phase. After liquid separat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com