Cellobiohydrolase mutant and application thereof
A cellobiose, hydrolase technology, applied in the directions of hydrolase, application, glycosylase, etc., can solve the problems of limited application, high cost, low efficiency of natural cellulose substrates, etc., to reduce enzyme dosage and improve conversion. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The wild-type cellobiohydrolase in this example is derived from Hypocrea jecorina, its amino acid sequence is shown in SEQ ID NO: 1, and its DNA molecular sequence is shown in SEQ ID NO: 2. Gene synthesis. The first and last 6 bases of SEQ ID NO: 2 and SEQ ID NO: 4 are restriction sites for NdeI (5') and XhoI (3').
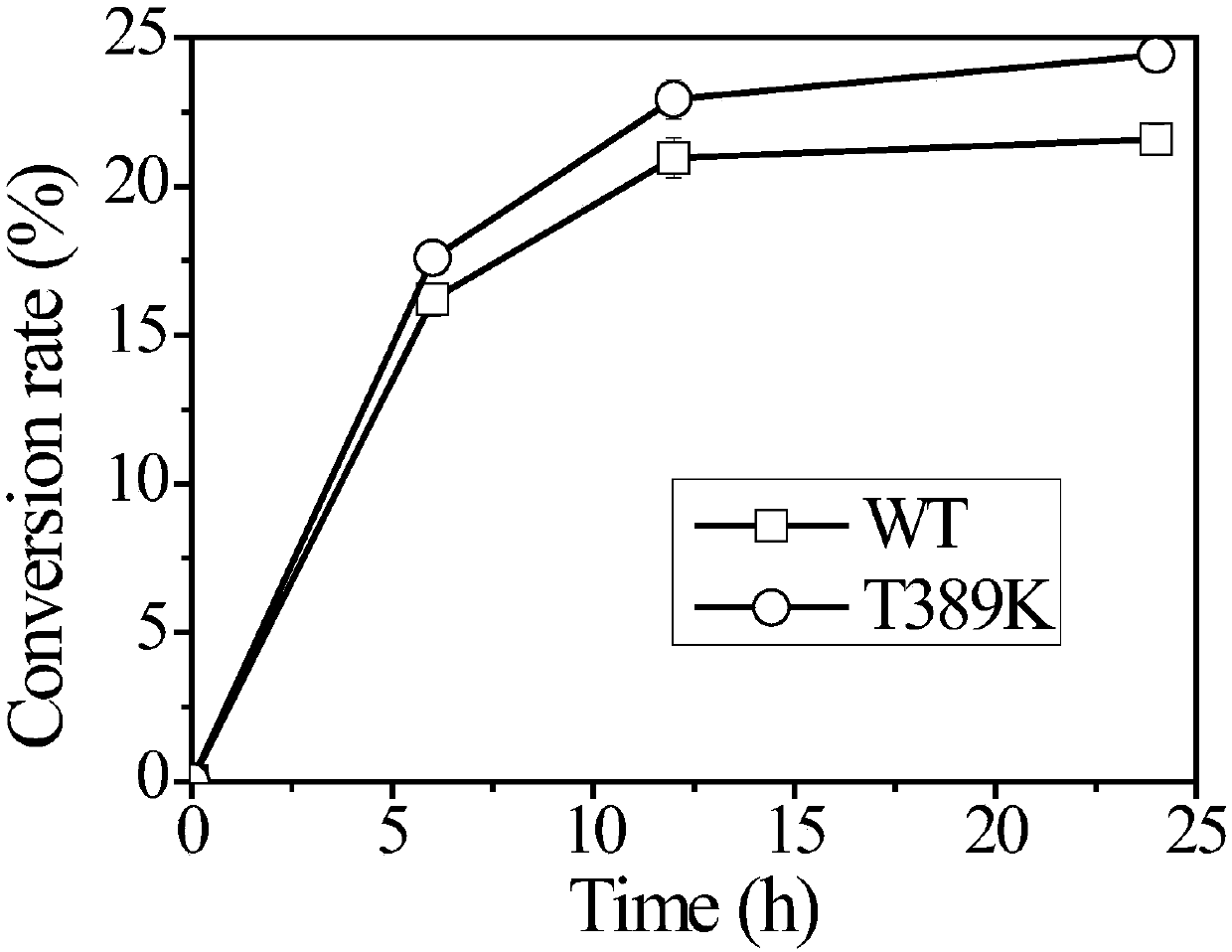
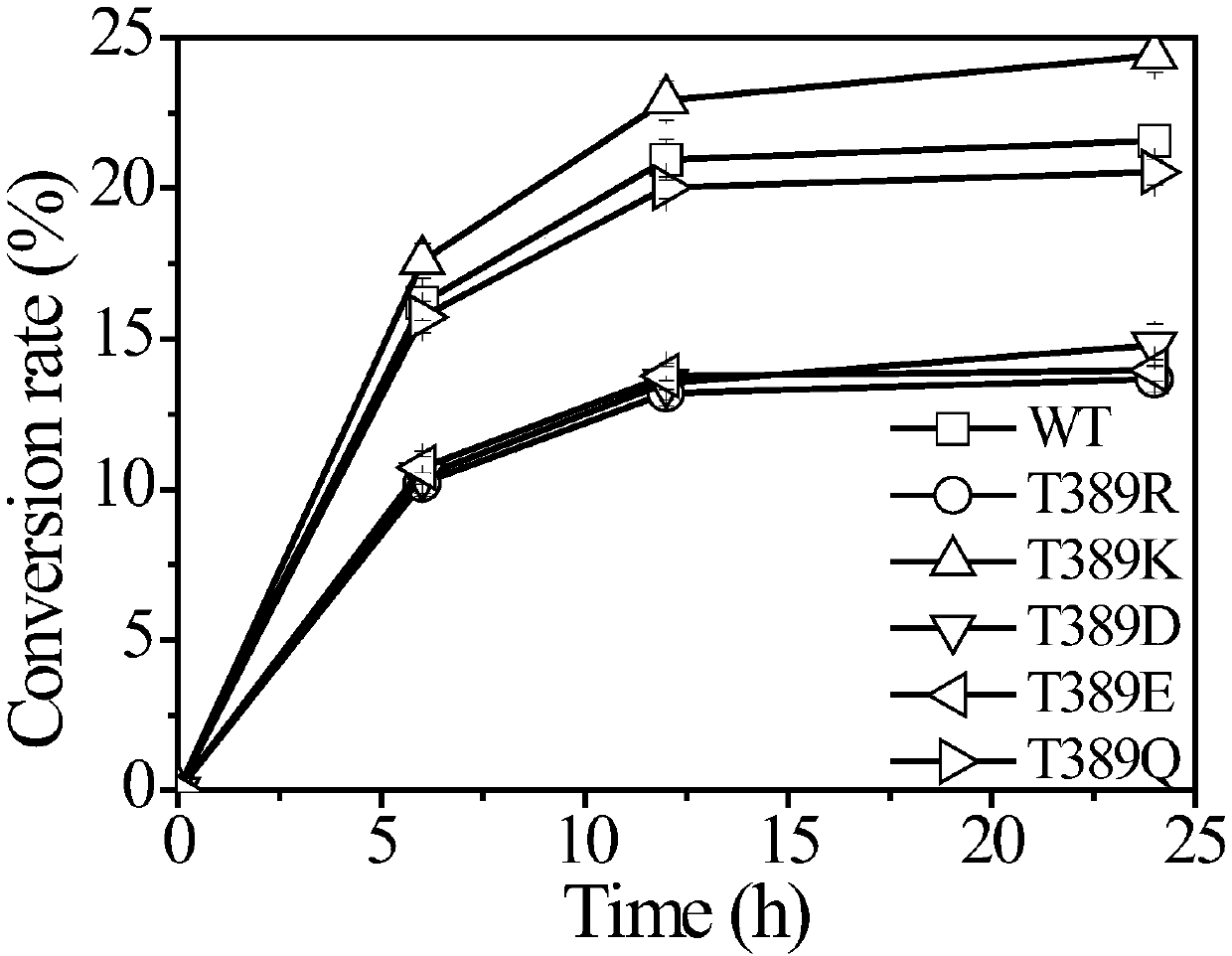
[0059] The cellobiohydrolase mutant provided by the present invention is obtained by mutating the protein encoded by the DNA molecule shown in SEQ ID NO:2. The recombinant plasmid containing the cellobiohydrolase DNA sequence (SEQ ID NO: 2) was used as a template for mutation to obtain cellobiohydrolase mutants T389K (SEQ ID NO: 3), T389R (SEQ ID NO: 5), T389D (SEQ ID NO: 7), T389E (SEQ ID NO: 9) and T389Q (SEQ ID NO: 11).
[0060] The expression vector used in the above construction method refers to pET15, pET22 or pET28 and the like.
Embodiment 2
[0062] 1. Construction of pET28a(+)-bgl1: a recombinant vector encoding the gene (nucleotide sequence SEQ ID NO:2) of wild-type cellobiohydrolase (amino acid sequence SEQ ID NO:1)
[0063] After the wild-type gene sequence (SEQ ID NO: 2) and an expression vector pET28a of the pET series were digested with NheI and XhoI enzymes, the digested gene fragment and the pET vector were purified and recovered, and the gene fragment was ligated The linker pET28a(+)-bgl1 was obtained on the pET28a vector. Transform the linker pET28a(+)-bgl1 into Escherichia coli E.coli DH5α, verify whether it is a correct gene clone (identical to the nucleotide sequence SEQ ID NO:2) to the obtained transformant sequencing, and select the correct sequence containing After the corresponding bacterial strain E.coli BL21(DE3) / pET28a(+)-bgl1 was cultivated in large quantities, the plasmid was extracted to obtain a large amount of correct recombinant vectors of pET28a(+)-bgl1 (amino acid sequence SEQ ID NO:1, ...
Embodiment 3
[0079] Expression and protein purification of genetic engineering bacteria containing cellobiohydrolase mutation of the present invention
[0080]The cellobiohydrolase mutant (nucleotide sequence SEQ ID NO:3) was inoculated in 20 mL of liquid LB medium containing kanamycin at a volume ratio of 2%, and cultured overnight at 28°C. Inoculate the activated culture solution into 100mL LB liquid medium containing kanamycin, place it at 37°C and 250rpm and cultivate it until OD600=0.6 (with UNICOUV2102 ultraviolet-visible spectrophotometer, the LB medium for culture is Blank control); then add IPTG with a final concentration of 0.5mM for induction, and continue to cultivate for 10 hours at 28°C and 180rpm; collect the bacteria by centrifugation at 4°C and 8000g, and add 0.1 times the volume of bacterial liquid binding buffer (Binding buffer), 350W power, ultrasonic 40min under the condition of ice bath to disrupt the cells, centrifuge at 30000g to collect the supernatant to obtain th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com