Preparation method of ion liquid crystal/polyimidazole semi-interpenetrating network polymer electrolyte
A semi-interpenetrating network, ionic liquid crystal technology, which is applied in the field of preparation of all-solid polymer electrolytes, can solve the problems of high investment cost and low energy consumption, and achieves the effects of simple and convenient operation, fast polymerization speed, and suitability for industrial applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
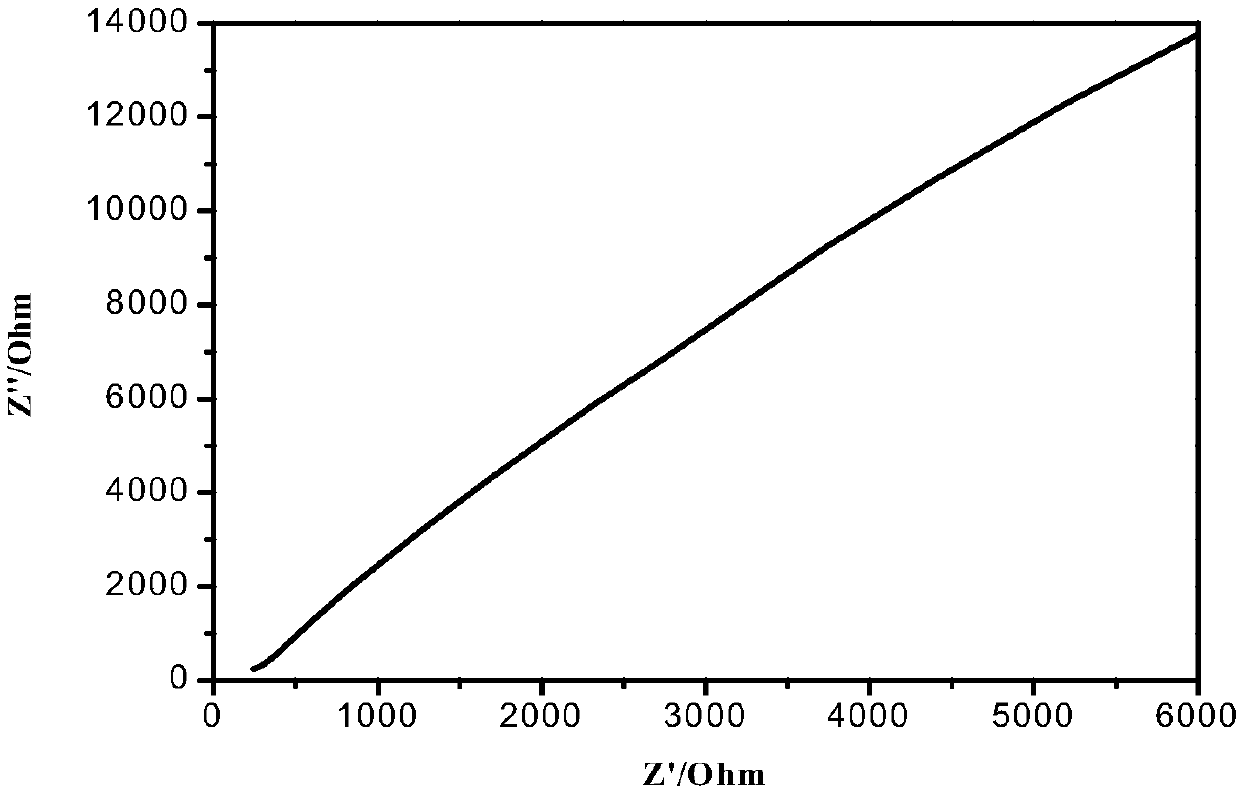
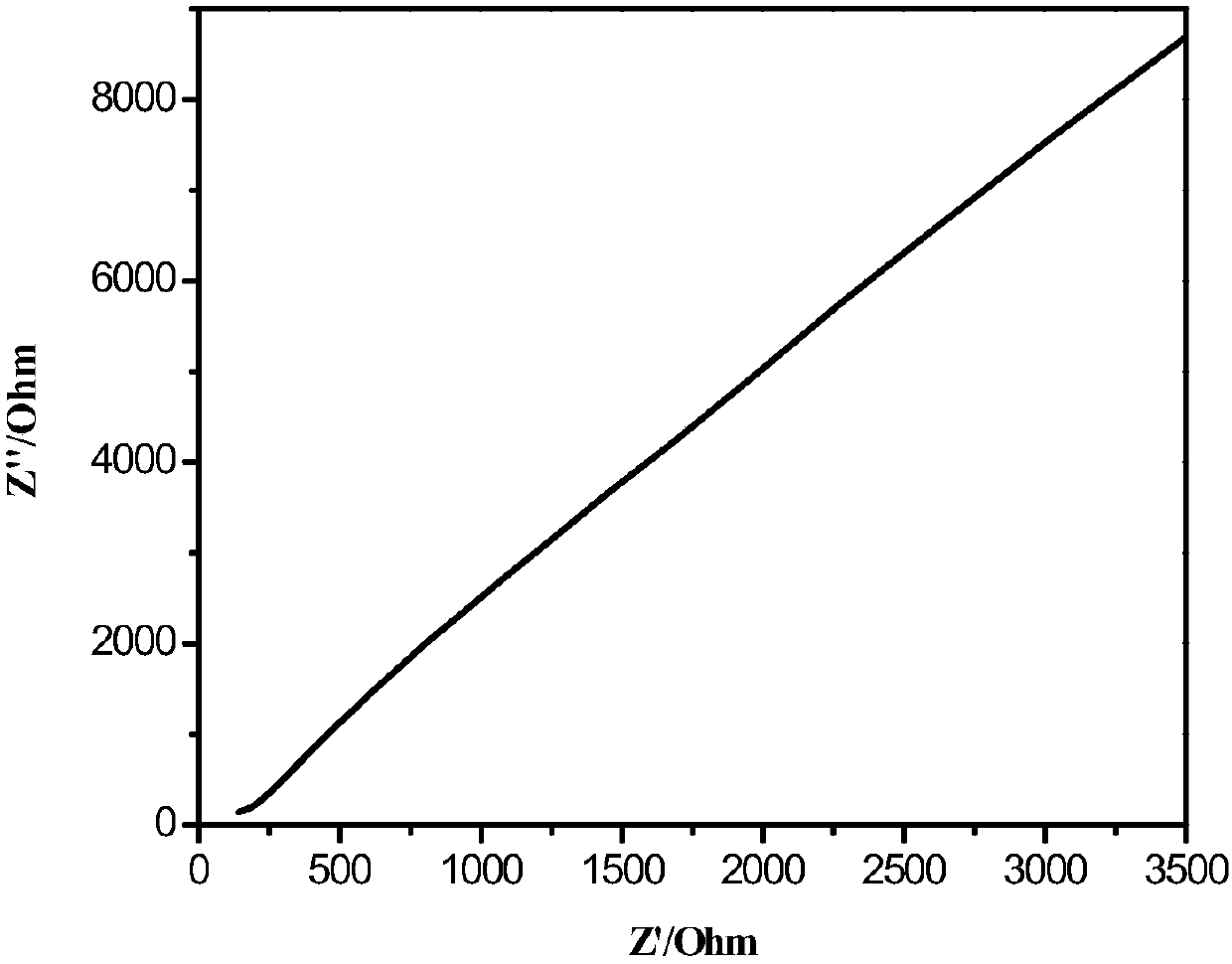
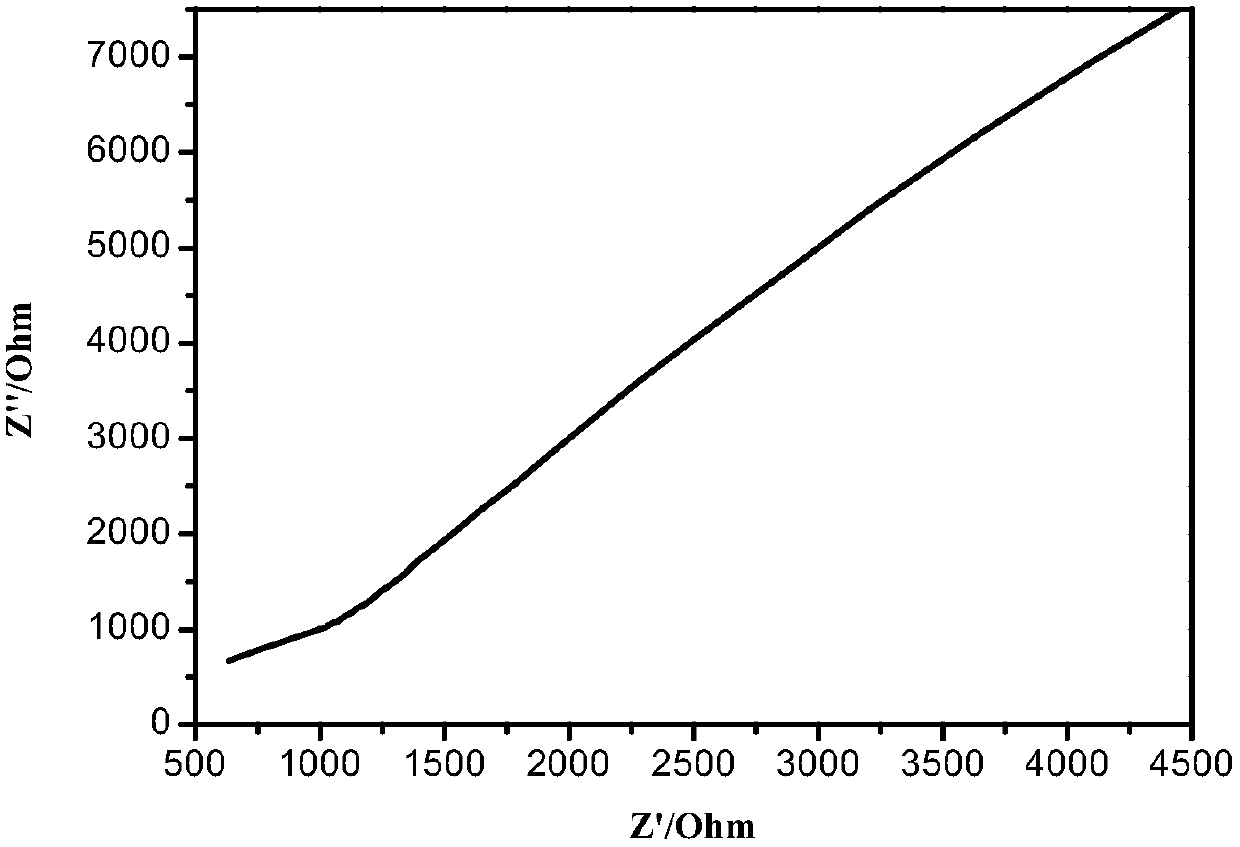
Image
Examples
Embodiment 1
[0039] Preparation of n-hexyl methacrylate-3-butylimidazolium bromide (MOBIm-Br):
[0040] Under the condition of ice bath, in a 250mL three-necked flask with a stirring bar, under N 2 Under the atmosphere, add 9g of 6-bromo-n-hexanol mixed solution dissolved in 30mL of dichloromethane, after stirring for 0.5h, slowly add 5.25g of triethylamine mixed solution dissolved in 30mL of dichloromethane (about 1 drop / second), add After stirring for 0.5h, add dropwise 4.9g of methacryloyl chloride dissolved in 30mL of dichloromethane and stir for 0.5h, then remove the ice area. Stir at room temperature for 18h. After the reaction was completed, suction filtered, and then 30 mL of deionized water was added to wash the filtrate for 4 times. Remove the water layer, add 3g of anhydrous magnesium sulfate to the organic layer for drying, after suction filtration, pour the liquid into a 250mL single-necked round-bottom flask and spin dry under vacuum, and spin dry with solvent to prepare 1-...
Embodiment 2
[0042] Imidazole liquid (MOBIm-BF 4 ) preparation:
[0043] (1) Put 29.7g of 1-n-hexyl methacrylate-3-butylimidazolium bromide in a round bottom flask, add 100mL of deionized water and stir. 7.45g LiBF 4 Dissolve in 30mL of water, add a stir bar and place on a magnetic stirrer to stir for 1h. Drop LiBF 4 The solution was slowly added dropwise to the MOBIm-Br solution. The reaction was stirred at room temperature for 5h.
[0044] (2) After the reaction is complete, extract the organic phase containing the ionic liquid with dichloromethane, and wash the resulting organic phase with deionized water until there is no Br - So far (with 0.1g mol -1 Silver nitrate Ag 2 NO 3 solution testing). Afterwards, add anhydrous magnesium sulfate to remove water, filter with suction, and remove dichloromethane by rotary vacuum evaporation to obtain a brownish-yellow viscous liquid which is the product MOBIm-BF 4 .
Embodiment 3
[0047] To 0.25g MOBIm-BF 4 , 0.0417g[Cmim]BF 4 and 0.0323g LiBF 4 Add 2-hydroxyl-2-methylpropiophenone (1 drop, as an initiator), add 0.5mL acetonitrile and stir for 30min to photopolymerize, then add 0.0833g PEGDA and 2-hydroxyl-2-methylpropiophenone (1 drop, (as an initiator) was added into 0.5 mL of acetonitrile solution for blending, stirred for 30 min, and cured to form a film under ultraviolet light irradiation. Then put it in a vacuum oven for vacuum drying for 24 hours.
[0048] And the product prepared in Example 3 is designated as Sample 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com