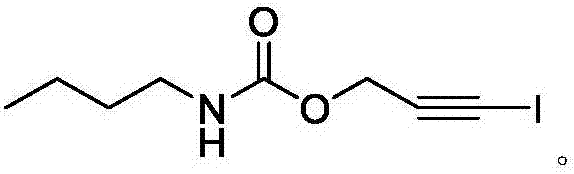
Preparation method for iodopropynyl butylcarbamate
A technology for butyl propynyl carbamate and propargyl ester, which is applied in the field of broad-spectrum fungicide preparation, can solve the problems of high reaction cost, safety and environmental pollution, complicated reaction steps, etc. The effect of high rate and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 A kind of preparation method of iodopropynyl butyl carbamate
[0017] 1) Weigh 58kg of sodium bicarbonate and 68kg of propyne bromide and dissolve them in 150L of nitrobenzene, then add them to the three-port reactor, after the addition is complete, heat up to 120°C, and keep the dropwise reaction temperature at 120°C to 140°C, continue After the reaction was completed for 36 hours, the organic solvent was directly filtered and distilled under reduced pressure to obtain 50 kg of 1-hydroxyformic acid-3-propynyl ester, and the yield (calculated as propyne bromide) was 88%.
[0018] 2) Weigh 75kg of potassium bicarbonate and 43kg of propyne chloride, dissolve them in 150L of dimethylformamide, add them to the three-port reactor, and raise the temperature to 120°C after the addition, and keep the dropwise reaction temperature at 120°C to 140°C , continue the reaction for 48h, and after the reaction is completed, directly filter and distill the organic solvent u...
Embodiment 2
[0022] 1) Weigh 68kg of 1-hydroxyformic acid-3-propynyl ester and 45kg of 1-butylamine and dissolve it in 150L of dimethoxydimethyl ether, add it to the three-port reactor, and after the temperature drops to 0-5°C, Slowly add 16kg of concentrated hydrochloric acid solution, the temperature rises to 120°C after the addition, and the reaction time is 12h. After the reaction is completed and the temperature drops to 5-10°C, continue to add 11kg of sodium iodide, and the temperature is raised to 25-30°C after the addition, and the reaction is continued for 24h , the resulting reaction solution was distilled under reduced pressure to remove the solvent, added an excess of 800mL of water and stirred for 30 minutes, filtered, and dried to obtain 157kg, iodopropynyl butyl carbamate, and the yield (in terms of 1-butylamine) was 91 %.
[0023] 2) Weigh 60kg of 1-hydroxyformic acid-3-propynyl ester and 37kg of 1-butylamine, dissolve them in 150L of diisopropyl ether, and add them into th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com