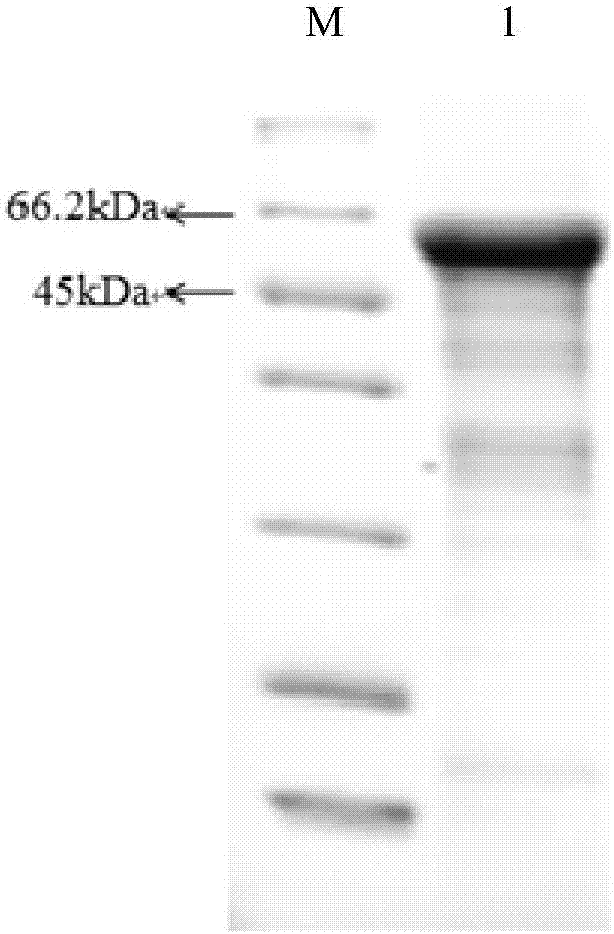
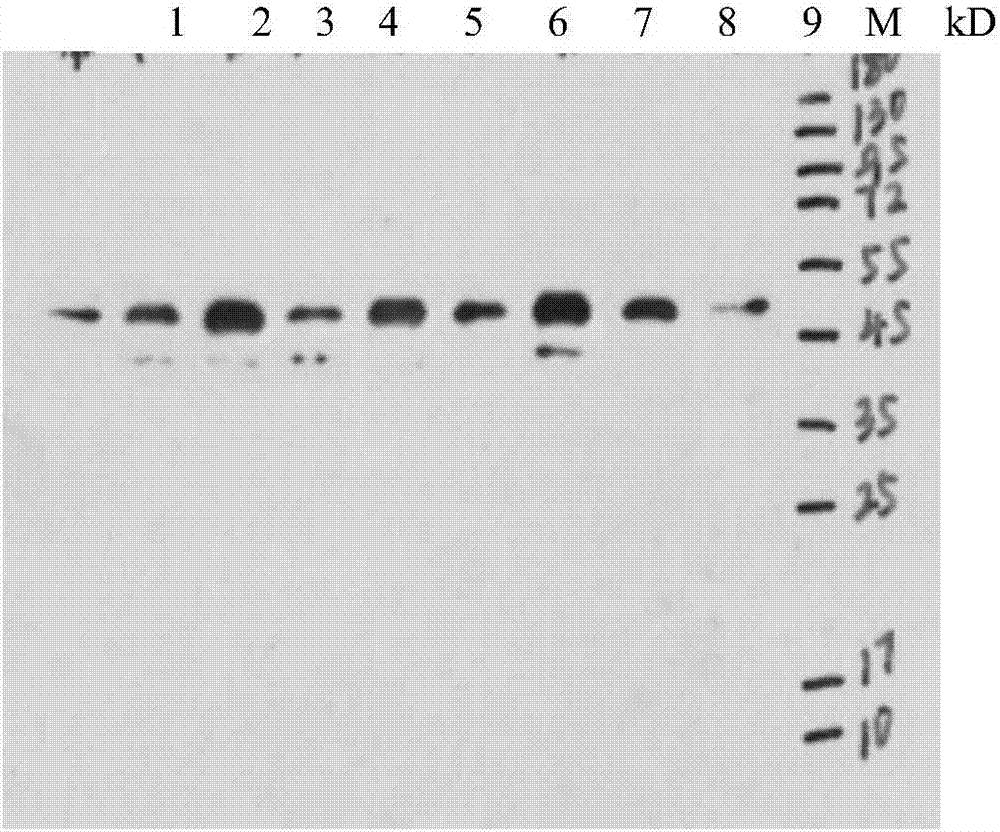
Determination method of effective antigen contents of infectious spleen and kidney necrosis virus inactivated vaccine and kit
A technology for spleen-kidney necrosis virus and inactivated vaccines, which is applied in the field of determination method and kit for the effective antigen content of inactivated vaccines for infectious spleen-kidney necrosis virus, and can solve problems such as practicability and biosafety drawbacks, antigen quantification, and virus diffusion , to achieve the effect of improving vaccine production efficiency, shortening the required time, and ensuring the quality of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Proliferation and purification of embodiment one ISKNV
[0032] CPB cells grew to the mid-logarithmic growth phase, inoculated with ISKNV at an MOI of 0.2, the final serum concentration was 6%, and cultured at a constant temperature at 28°C. When the cells produced 90% of the lesions, the virus was collected. Virus purification by density gradient centrifugation.
[0033] (1) Virus precipitation: collect the virus-infected cell suspension, centrifuge at 7500rpm 4°C for 30min; take the supernatant and centrifuge at 150,000×g (about 28000rpm) at 4°C for 1h (BECKMAN 70Ti), discard the supernatant; add appropriate amount of TN buffer, pipette, resuspend the pellet, (at this time, the pellet is often agglomerated, which is not conducive to the gradient centrifugation below), and ultrasonically break several times until no obvious granular precipitate appears.
[0034] (2) Over a sucrose cushion: Add the precipitated virus suspension on top of 35% sucrose solution, centrifug...
Embodiment 2
[0038] Embodiment 2 Recombinant protein expression and purification
[0039] The ISKNV mcp gene fragment was connected to the pET-SUMO vector, and the correct recombinant plasmid was identified by sequencing to transform the expression host. Pick a single colony containing the recombinant plasmid and culture it overnight at 37°C in 3ml LB (containing antibiotics). Take 30 μL of the overnight culture solution and add it to 20 mL of LB medium, shake and culture at 37°C until OD 600 About 0.6, part of the liquid was taken as the uninduced control group, and the rest was added with IPTG inducer to a final concentration of 0.5mM as the experimental group, and the two groups continued to culture with shaking at 37°C for 3h. The fermentation broth was collected, and the cells were collected by centrifugation at 6000g for 10min. Suspend the cells in 40 mL of pre-cooled NTA-0 buffer. Bacteria were crushed by ultrasonic waves in an ice bath, the control power was 300W, ultrasonic wav...
Embodiment 3
[0041] The screening of embodiment tri-hybridoma cell line
[0042] Use the mixture of ISKNV-QY strain purified virus and recombinant ISKNV MCP protein as antigen, immunize BalB / c mice by intraperitoneal cavity, 50-100 μg / mouse for the first immunization, the adjuvant is complete Freund's adjuvant, boost immunization mice 50- 80 μg / monkey, the adjuvant is incomplete Freund's adjuvant, a total of four immunizations, the first immunization interval is 2-3 weeks, and then the booster immunization is 2 times with an interval of 1 week, and then the titer is tested, and it is higher than 1:10000 after 1 week Intraperitoneal percussion. Three days after the last impact, the eyeballs were removed and sacrificed, the positive control blood was collected, the spleen was taken out, and a single cell suspension was prepared, and the splenocytes were fused with SP2 / 0 cells with PEG1450 (1:5-1:10); detected by indirect ELISA The antibody titer in the supernatant of hybridoma cells, select...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com