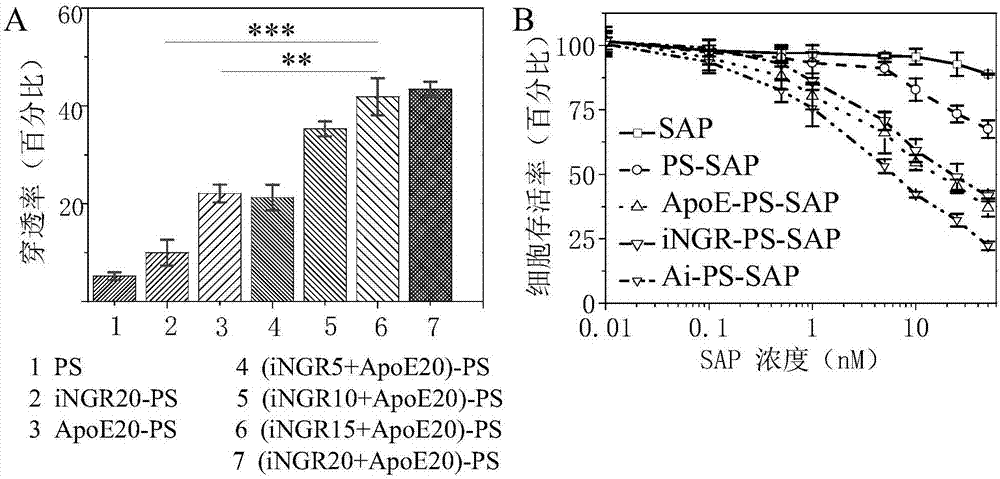
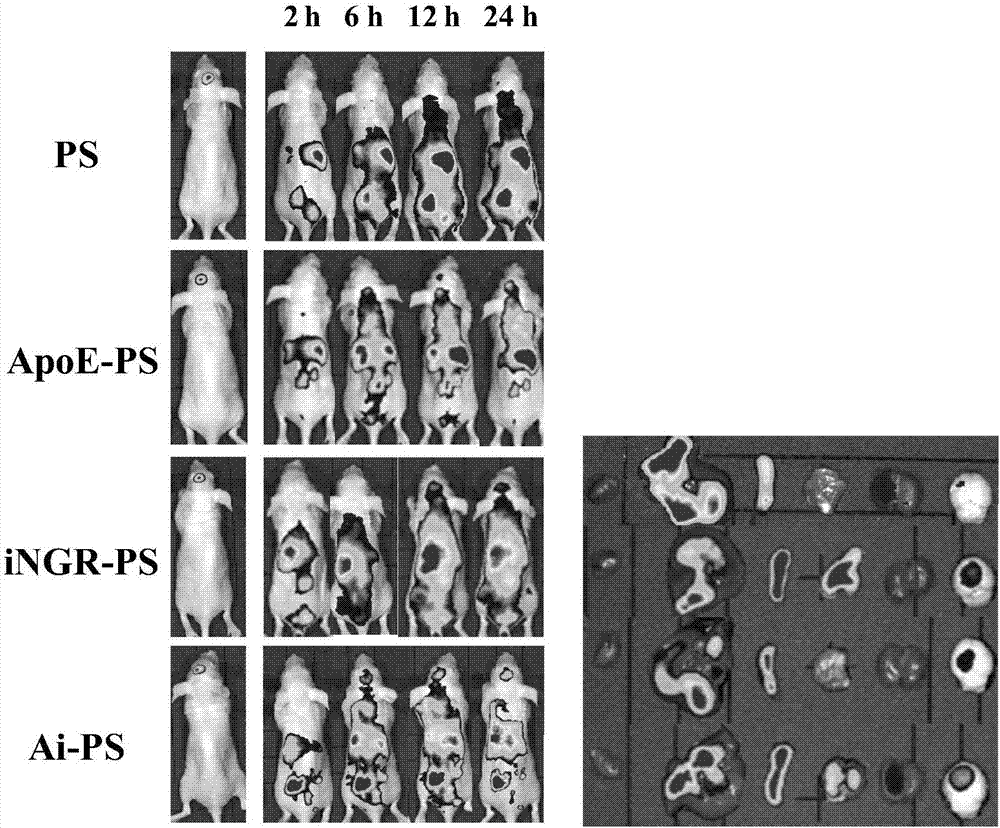
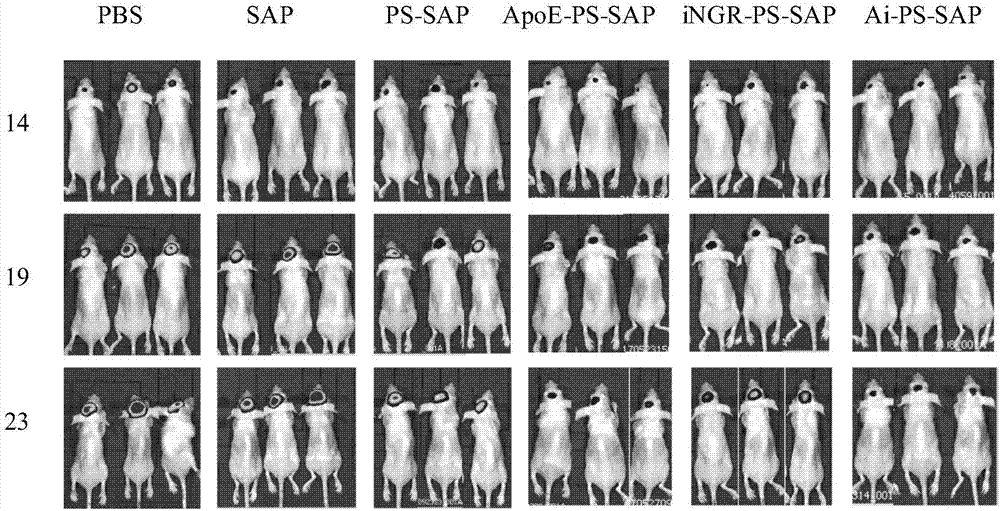
Application of reduction response polymersome nano-drug in preparation of brain tumor treatment drugs
A technology of nano-drugs and therapeutic drugs, which is applied in the direction of anti-tumor drugs, drug combinations, and non-active ingredients of polymer compounds, which can solve the problems of limiting the therapeutic effect of brain tumors and the limited penetration depth of brain tissue, so as to reduce the adsorption of proteins , enrichment and drug release rate increase, the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Synthesis of block copolymers PEG5k-P (DTC2k-TMC15k) and PEG5k-P (DTC2k-TMC15k)-bPEI1.8k
[0048] In the nitrogen glove box, weigh out MeO-PEG-OH ( M n = 5.0 kg / mol, 0.50 g, 100 μmol), TMC (1.52 g, 14.55 mmol) and DTC (0.23 g, 1.18 mmol) and dissolved in dichloromethane (DCM, 7.0 mL), stir and quickly add the catalyst diphenyl phosphate Ester (DPP, DPP / OH molar ratio is 10 / 1). The airtight reactor was sealed and placed in a 40 degree oil bath under magnetic stirring to react for 2 days. After termination of triethylamine, precipitation in ice ether twice, suction filtration, and vacuum drying, PEG5k-P (DTC2k-TMC15k) was obtained.
[0049] PEG5k-P (DTC2k-TMC15k) terminal hydroxychloroformic acid p-nitrophenyl ester NPC activation, and then with branched PEI (bPEI) primary amine to prepare. Specifically, PEG5k-P (DTC2k-TMC15k) (0.4 g, hydroxy 0.017 mmol) and NPC (50 mg, 0.09 mmol) were dissolved in dry DCM and reacted at 0°C for 24 hours, then precipitated in ice e...
Embodiment 2
[0051] Example 2 Synthesis of Targeting Polymer
[0052] There are many ways to synthesize targeting polymers, depending on the functionalized end groups of PEG. The target diblock polymer ApoE-PEG7.5k-P (DTC4.4k-LA19.8k) is synthesized in two steps. The first step is similar to the synthesis of PEG5k-P (DTC4.4k-LA19.8k). First, Use Mal-PEG-OH (Mn = 7.5 kg / mol) instead of MeO-PEG-OH ( M n = 5.0 kg / mol) initiates the ring-opening polymerization of DTC and LA to obtain Mal-PEG7.5k-P (DTC4.4k-LA19.8k). The second step is to react according to the peptide ApoE (sequence LeuArg Lys Leu Arg Lys Arg Leu Leu Arg Lys Leu Arg Lys Arg Leu Leu Cys) and Mal-PEG7.5k-P (DTC2k-LA15k) at a ratio of 1.2:1, Under nitrogen, ApoE dissolved in DMSO was added dropwise to Mal-PEG7.5k-P (DTC2k-LA15k) dissolved in DMSO, and the reaction was stirred at 37°C for 8 hours. After dialysis with DMSO for 24 hours and then with water for 12 hours, ApoE-PEG7.5k-P (DTC4.4k-LA19.8k) was obtained by freeze-drying. ...
Embodiment 3
[0057] Example 3 Synthesis of block polymer PEG5k-P(TMC15k-DTC2k)-Sp
[0058] PEG5k-P(DTC2k-TMC15k)-NPC, synthesized by the same method as in Example 1, was dissolved in 3 mL DCM, then added dropwise to 3 mL DCM with spermine (26 mg, 0.13 mmol), and reacted at 30°C for 48 hours After that, it was dialyzed (MWCO 7000) in DCM and methanol (volume ratio 1:1) for 48 hours, precipitated with ice ether twice, filtered with suction, and dried in vacuum to obtain PEG5k-P(DTC2k-TMC15k)-Sp. Yield: 94.7%. NMR and TNBSA methods characterize the Sp grafting rate of 97%. Table 1 lists the preparation conditions of each polymer and the NMR characterization results of the product, and the targeting molecule can be attached through the linking group.
[0059] Table 1 Preparation conditions and NMR characterization results of each polymer
[0060]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com