Expression and purification method of phospholipase A1 accessory protein PlaS
An auxiliary protein and expression method technology, applied in the field of molecular biology, to achieve the effect of relaxed experimental environment requirements, simple methods, and large protein adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
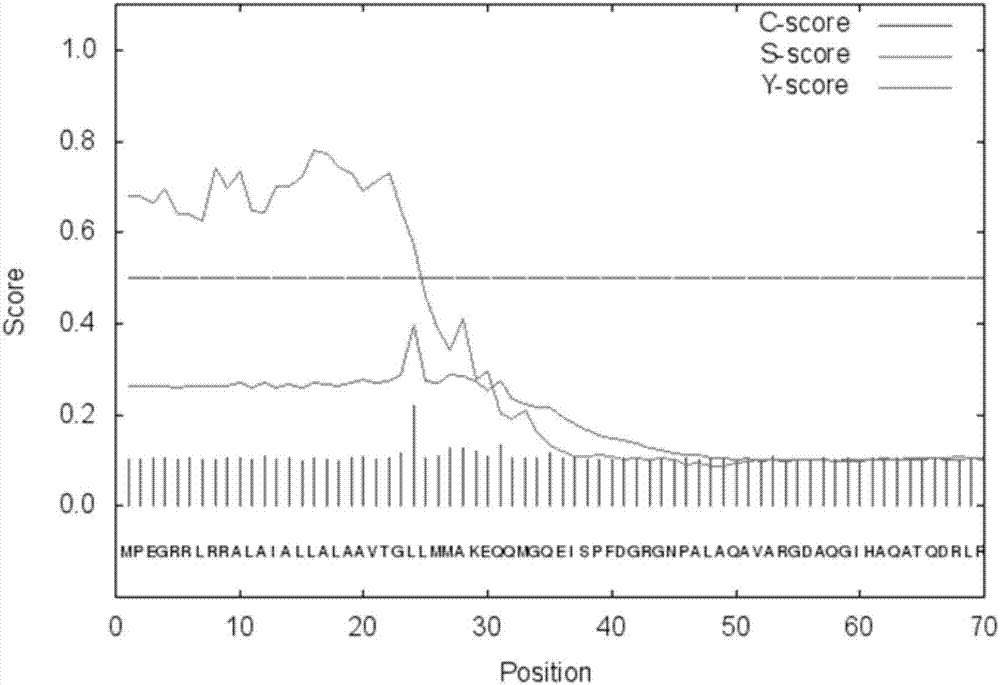
[0031] Based on the bioinformatics analysis of the phospholipase A1 auxiliary protein plaS gene sequence (the analysis was provided by Detai Biotechnology (Nanjing) Co., Ltd.), the inventors found that there is a signal peptide at the front end, and the first 35AA is the signal peptide region ( figure 1 ),in:
[0032] figure 1 It is the analysis result of signal peptide of phospholipase A1 auxiliary protein plaS gene.
[0033] Therefore, the inventors used Primer 5.0 to design N-terminal truncated primers for plaS:
[0034] upstream primer P1CG GGATCC GAGATTTCACCGTTTG
[0035] downstream primer P2CCC AAGCTT CTGCTGCGCGTAGT
[0036] Among them, the underlined parts are the restriction sites of BamHI and HindIII respectively.
[0037] Specific steps are as follows:
[0038] Using the BL21 / SP28 previously constructed in our laboratory, that is, the plasmid of the recombinant plasmid plaS-pET-28 constructed in Chinese patent CN103333229, as a template, and P1 and P2 as pr...
Embodiment 2
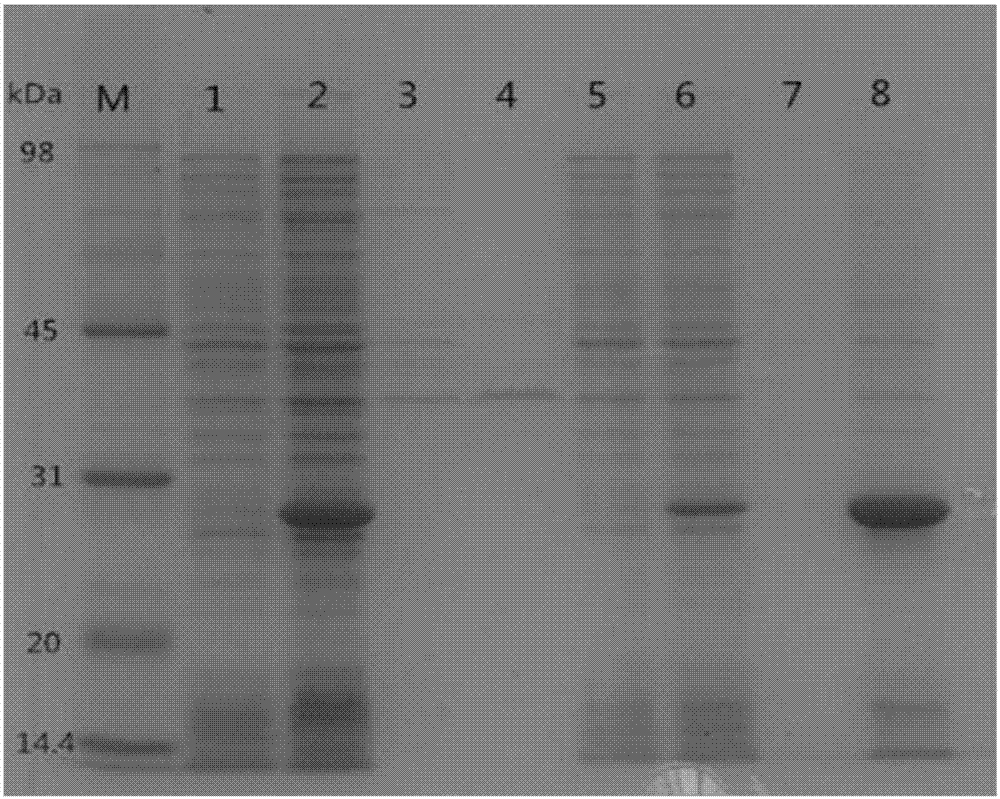
[0052] The dSP28 bacterial strain successfully constructed in Example 1 is in LB medium, 37 DEG C, 200r / min cultured overnight, next day by 2 (V / V)% inoculum size seed liquid is inoculated to 100mL plus kanamycin (working Concentration of 50μg / mL) in the LB fermentation medium, until the concentration of bacteria to OD 600nm At about 0.7, add IPTG to induce 8h, prepare protein samples, and observe protein expression sites ( figure 2 ).
[0053] figure 2 It is the SDS-PAGE of P28 and dSP28, wherein, dSP28 is fermented with LB fermentation medium added with kanamycin and induced by IPTG.
[0054] Among them, 1, 3, 5, and 7 are the whole bacteria, fermentation broth supernatant, broken supernatant, and broken bacterial protein of P28 respectively, and 2, 4, 6, and 8 are the whole bacteria of dSP28, fermentation broth supernatant protein, broken supernatant protein, broken cell protein.
[0055] SDS-PAGE protein electrophoresis sample preparation for preliminary identificati...
Embodiment 3
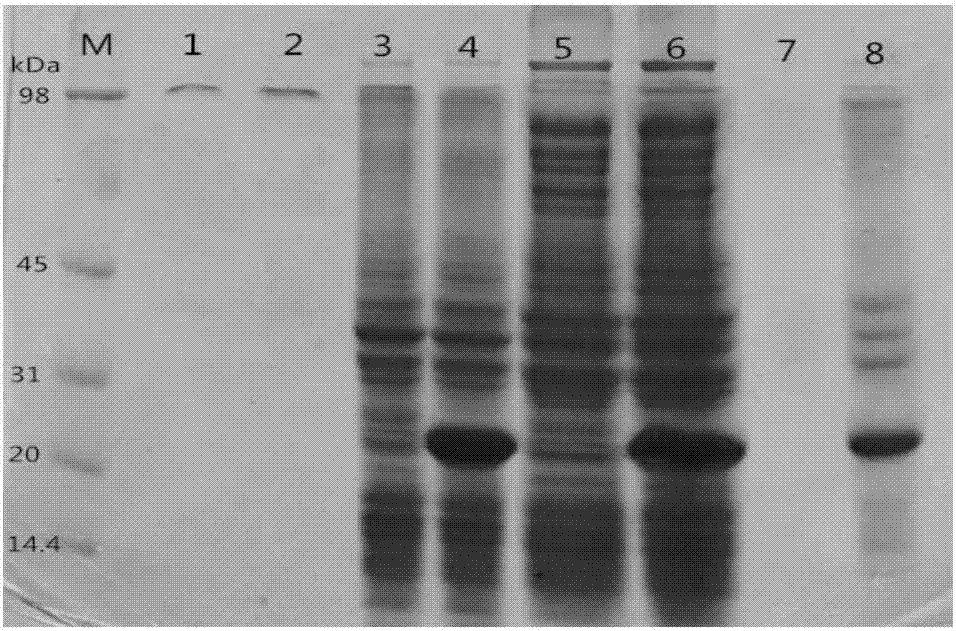
[0085] The successfully constructed dSP28 strain in Example 1 was cultured overnight in LB seed solution, and the next day, the seed solution was inoculated into media of different components at an inoculum size of 2% (V / V) to induce expression of the target protein, and prepared protein samples, and observe the expression level of the target protein (Figure 3).
[0086] The different media are as follows:
[0087] a. TB culture medium;
[0088] b. TB medium plus 0.1wt% SDS;
[0089] c. TB medium plus 0.5wt% Tween 20;
[0090] d. TB medium plus 0.98wt% glycerol and 0.75wt% glycine;
[0091] e. TB medium plus 3wt% glycerol;
[0092] f. Lactose self-induction medium;
[0093] g. Add 0.75wt% glycine 6h after self-induction of lactose.
[0094] The above-mentioned five kinds of fermentation media a, b, c, d, and e were inserted into the seed liquid, and then cultivated at 37°C and 200r / min for 2h, that is, OD 600nm At about 0.7, add 0.2mM IPTG, and ferment for 22 hours at 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com