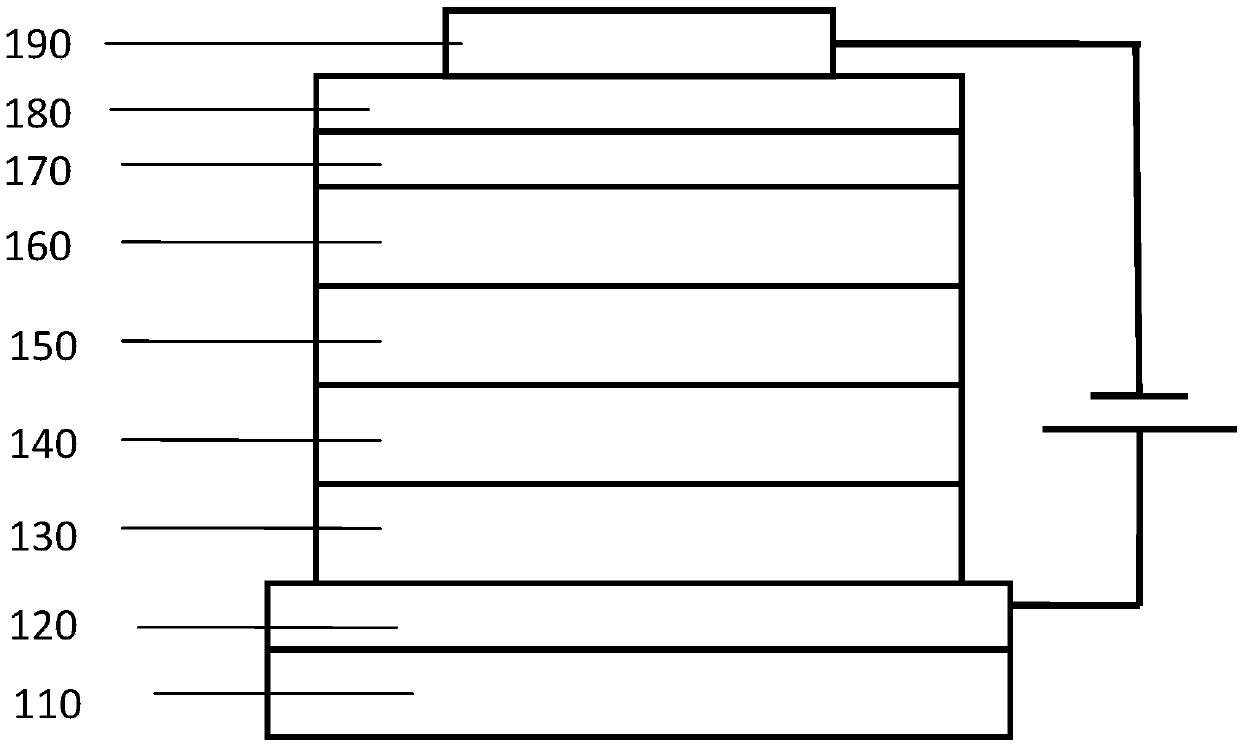
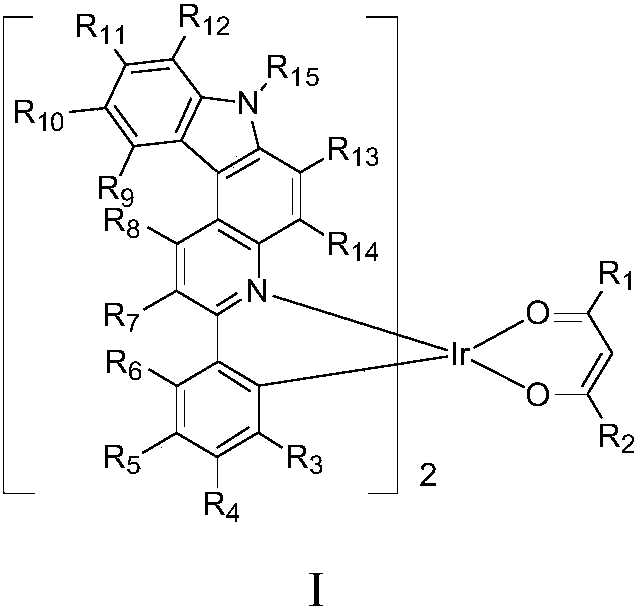
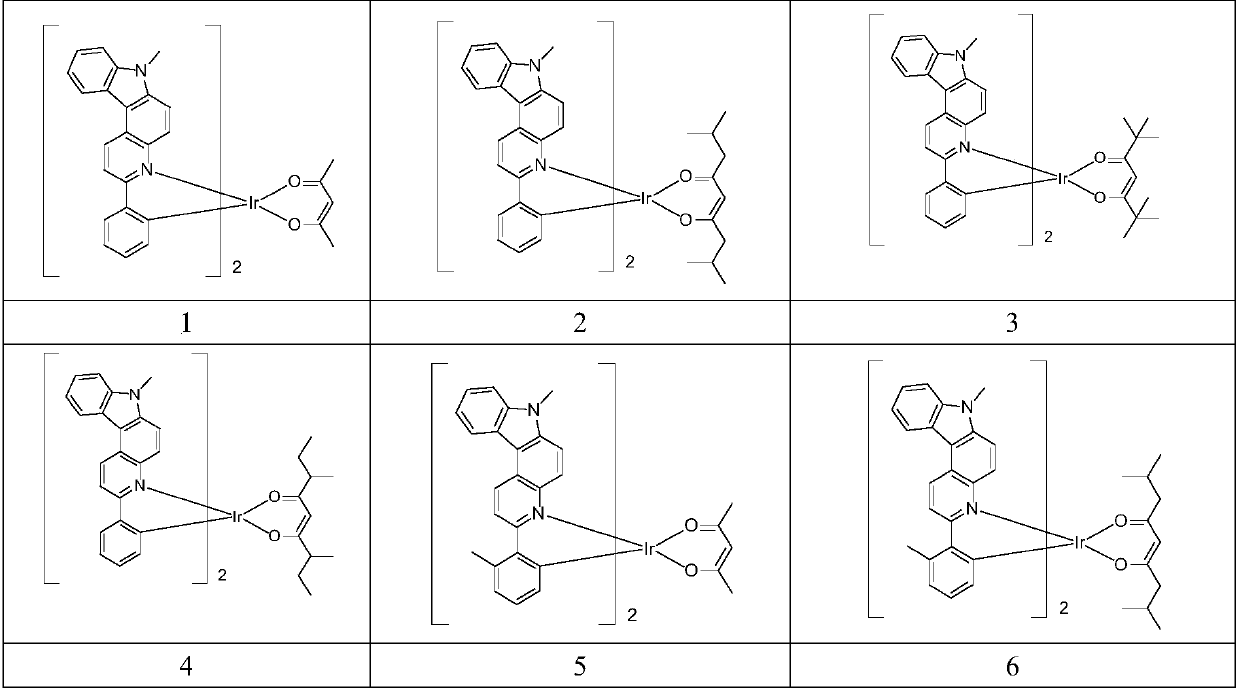
Iridium complex, application thereof and OLED (organic light-emitting device)
A technology of iridium complexes and compounds, which is applied in the field of organic electroluminescent devices, can solve problems affecting device life and efficiency, and organic materials are prone to crystallization, and achieve good thermal stability, high luminous purity, and high luminous efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthetic route of compound 1
[0049]
[0050] The synthetic method of intermediate 1-1
[0051] In a flask, add 3-amino-9-methylcarbazole (10g, 51mmol), 3,5-dimethylbenzaldehyde (5.4g, 51mmol), trimethylsilylacetylene (5g, 51mmol), trifluoro Lanthanum methanesulfonate (1g), cuprous iodide (1g) and 30mL of 1-butyl-3-methylimidazolium tetrafluoroborate were reacted at 100°C for 5 hours, water was added, and extracted with dichloromethane , dried, concentrated, and the crude product was purified by column chromatography to obtain 11.5 g with a yield of 75%.
[0052] The synthetic method of intermediate 1-2
[0053] Add intermediate 1-1 (1.5g, 4.9mmol), iridium trichloride trihydrate (0.60g, 2mmol), water (5mL), ethoxyethanol (15mL) in a one-necked flask, and heat to reflux under nitrogen protection 24 hours. After the reaction was completed, it was cooled to room temperature, the solid was filtered out with suction, and the filter cake was washed with ethanol to o...
Embodiment 2
[0057] Synthetic route of compound 28
[0058]
[0059] The synthetic method of intermediate 28-1
[0060] Add 5-chloro-2-(3,5-dimethylphenyl) quinoline (5.34g, 20mmol), o-nitrophenylboronic acid (4.01g, 24mmol), potassium carbonate (6.90g, 50mmol) ), palladium acetate (0.11g, 0.5mmol), 2-dicyclohexylphosphonium-2,4,6-triisopropylbiphenyl (0.36g, 0.75mmol), dioxane (75mL) and water (25mL ), heated to reflux under nitrogen protection for 8 hours. Cool to room temperature, remove dioxane by rotary evaporation, extract the organic phase with dichloromethane, combine the organic phases, and dry with anhydrous sodium sulfate. The crude product was separated by column chromatography to obtain 3.15 g of a yellow solid with a yield of 43.7%.
[0061] The synthetic method of intermediate 28-2
[0062] Add intermediate 28-1 (2.12g, 6mmol), triphenylphosphine (4.72g, 18mmol), o-dichlorobenzene (25mL) into a single-necked flask, heat and reflux under nitrogen protection for 5 hours...
Embodiment 3
[0070] Synthetic route of compound 40
[0071]
[0072] The synthetic method of intermediate 40-1
[0073] In a flask, add 3-amino-9-ethylcarbazole (6g, 28.6mmol), 3,5-dimethylbenzaldehyde (3.8g, 28.6mmol), phenylacetylene (3g, 28.6mmol), trifluoro Lanthanum methanesulfonate (0.5g), cuprous iodide (0.5g) and 30mL of 1-butyl-3-methylimidazolium tetrafluoroborate were reacted at 100°C for 5 hours, water was added, and dichloro Extracted with methane, dried and concentrated, the crude product was purified by column chromatography to obtain 10 g with a yield of 84%.
[0074] The synthetic method of intermediate 40-2
[0075] The synthesis method is the same as that of intermediate 28-4, and the raw material used is intermediate 40-1.
[0076] The synthetic method of compound 40
[0077] The synthesis method was the same as that of compound 28, and the yield was 16%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com