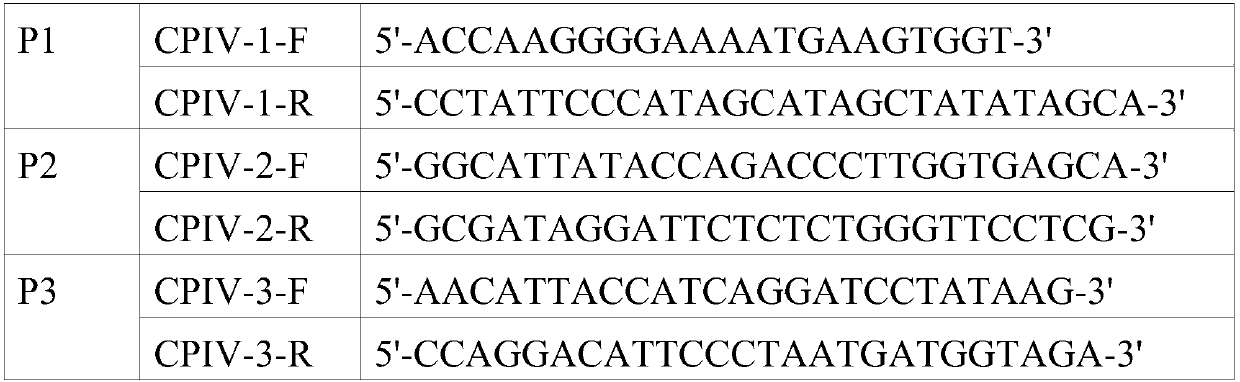Canine parainfluenza virus strain and application thereof
A technology of canine parainfluenza virus and canine parvovirus, which is applied in the direction of viruses, antiviral agents, and viral antigen components, can solve the problems of strong virulence, low immune efficacy of inactivated vaccines, and inability to completely resist infection. The effect of good biological security, good prevention and control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0028] As an embodiment of the present invention, the inactivating agent includes but not limited to β-propiolactone BPL, binary ethyleneimine BEI, formalin, formaldehyde, N-acetylethyleneimine AEI, polyhexamethylene hydrochloride Methylguanidine PHMG.
[0029] As an embodiment of the present invention, in the vaccine composition of the present invention, the culture of the canine parainfluenza virus strain is a culture cultured for 1-18 generations.
[0030] As an embodiment of the present invention, in the vaccine composition of the present invention, the culture of the canine parainfluenza virus strain is a culture cultured for 6-18 generations.
[0031] As an embodiment of the present invention, in the vaccine composition of the present invention, the adjuvant includes: (1) aluminum gel adjuvant, saponin, avridine, DDA; (2) water-in-oil emulsion, water Oil-in-emulsions, water-in-oil-in-water emulsions; or (3) polymers of acrylic or methacrylic acid, copolymers of maleic a...
Embodiment 1
[0049] The separation and identification of embodiment 1 canine parainfluenza virus
[0050] The wild strain of CPIV was isolated from clinically infected animals with canine parainfluenza virus. The clinical symptoms of the infected animals were: mental depression, fever, a large amount of mucus from the nasal cavity, opaque secretions, dry cough, etc., which were used as the criteria for the selected animals. The lung tissue of the infected animal was taken and ground with a tissue homogenizer to obtain a tissue homogenate.
[0051] Dilute the lung tissue homogenate of sick dogs to 10% W / V suspension with serum-free DMEM culture fluid, filter and sterilize with a 0.22 μm filter membrane, and inoculate the filtrate into Vero monolayer cells (purchased from Shanghai Enzyme Detection Technology Co., Ltd.) for virus isolation, cell fusion lesion appeared in 24 to 48 hours, and the cell lesion reached more than 80% in about 96 hours, and the freeze-thawed virus was collected as t...
Embodiment 2
[0052] Embodiment 2 canine parainfluenza virus clone
[0053] 1. Sequencing of the whole genome of canine parainfluenza virus
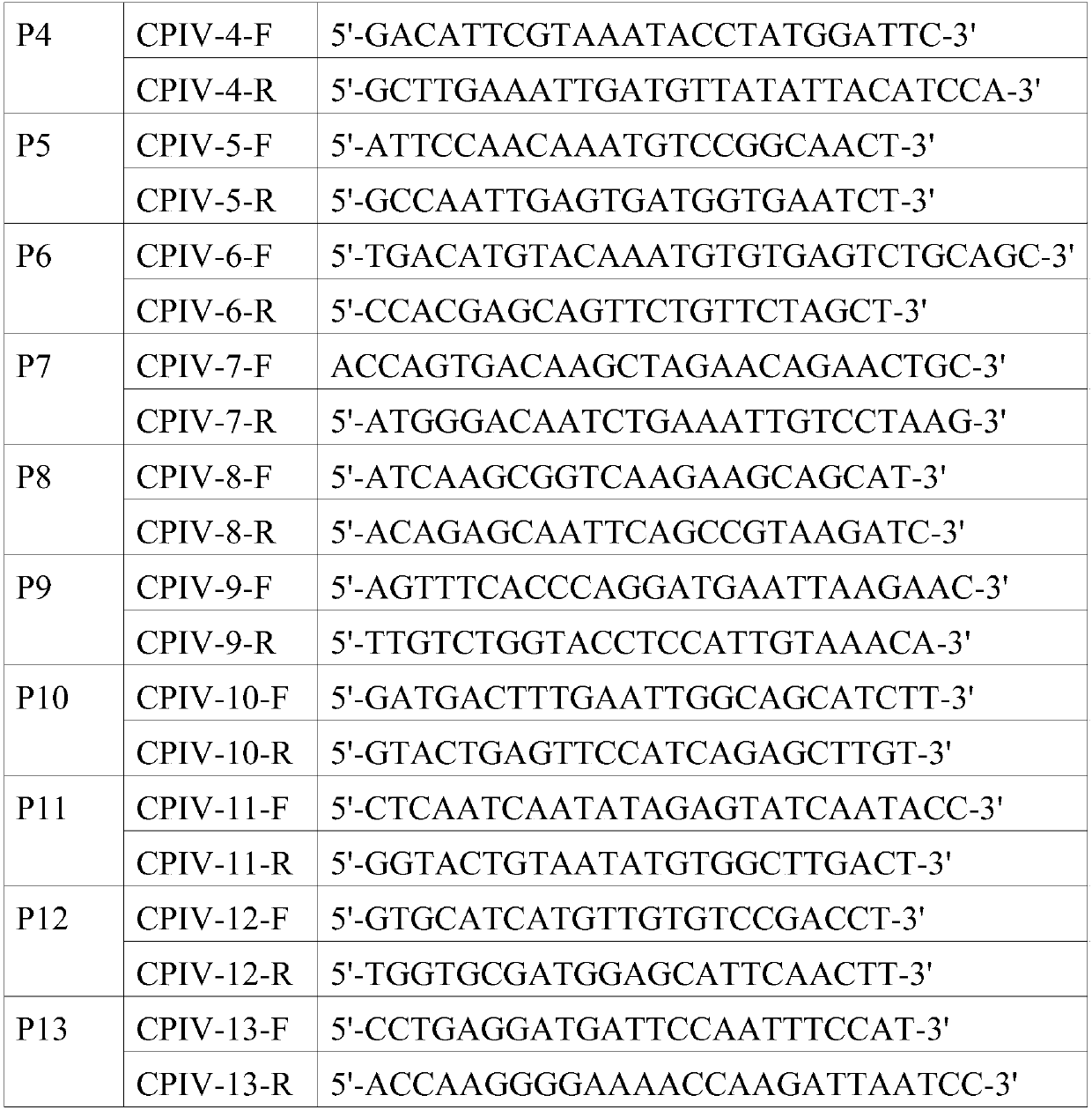
[0054] Referring to the CPIV5 sequence (JQ743318.1) submitted in GenBank, 13 pairs of primers were designed. The amplified fragments partially overlap with each other and can be connected to cover the whole genome. The primer sequences are shown in Table 1.
[0055] Table 1 Sequence list of canine parainfluenza virus whole genome sequencing primers
[0056]
[0057]
[0058] Referring to the instructions of the RNA extraction kit, extract the total RNA of the CPIV virus liquid isolated in Example 1, and use the downstream primers of P1 to P13 to perform reverse transcription reactions respectively to prepare the cDNA of the virus. Using the obtained cDNA as a template, use the designed 13 pairs of primers amplified the whole genome of the virus. After the PCR product was identified by 1% agarose gel electrophoresis, the size of the band was in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com