Novel iron ion fluorescence probe and preparation method thereof
A fluorescent probe, iron ion technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of few species and insignificant detection signals, and achieve high selectivity, obvious phenomenon, and simple preparation method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of 7-N,N-Diethylaminocoumarin-3-carbaldehyde
[0043] Add diethylamino salicylaldehyde (0.97g, 5mmol), diethyl malonate (1.6g, 10mmol), piperidine (1.0ml) into 15mL ethanol solvent, reflux at 120°C for 18h, reduce The solution was separated by suction filtration; 20ml of concentrated hydrochloric acid and 20mL of glacial acetic acid were added thereto, stirred at 115°C for 19h, cooled to room temperature, the resulting solution was poured into 50ml of ice water, and NaOH solution was added dropwise to adjust the pH to 5. The precipitate was obtained, stirred at room temperature for 1 h, filtered with water, washed and dried to obtain an intermediate product.
[0044] a. Add 7 mL of redistilled N,N-dimethylformamide to 7 mL of POCl 3 , stirred under nitrogen protection for 35min, the solution gradually turned into a red clear solution;
[0045] b. Dissolve the intermediate product obtained in step a in 30mL N,N-dimethylformamide, add dropwise to the red c...
Embodiment 2
[0049] Preparation of Schiff base compounds (fluorescent probes for iron ions based on coumarin derivatives)
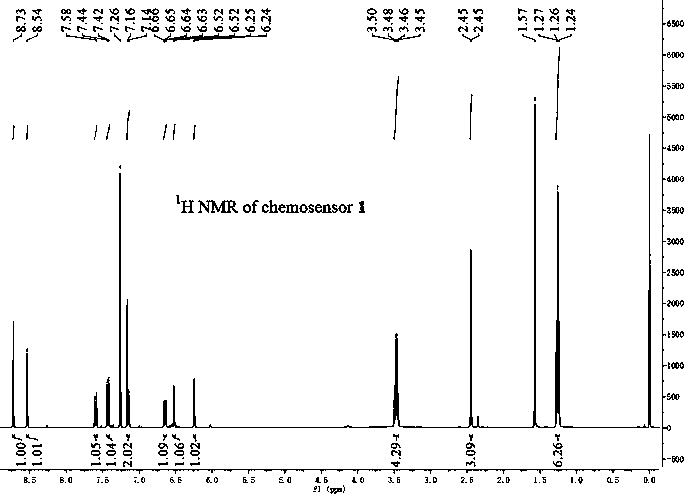
[0050] 7-N, N-diethylaminocoumarin-3-carbaldehyde (0.74g, 3mmol) and 7-amino-4-methylcoumarin (0.52g, 3mmol) were mixed at a molar ratio of 1:1 Put it into a beaker and a round-bottomed flask filled with absolute ethanol, heat the round-bottomed flask filled with 7-amino-4-methylcoumarin to 70°C, and after 3min, add 7-N,N-di Ethylaminocoumarin aldehyde was added to the round bottom flask within 1 min. After the color of the system turned orange, 3 drops of glacial acetic acid were added dropwise. After reacting for 10 h, it was cooled to room temperature, and then placed in the refrigerator for 10 min to precipitate Precipitate, filter, wash with organic solvent, and dry to obtain the target product with a yield of 64%. The resulting compound was subjected to nuclear magnetic resonance spectrum analysis, 1 H NMR (400MHz, CDCl 3 )δ: 1.26 (t, J = 7.2Hz, 6H), 2.45(d...
Embodiment 3
[0054] Detection of Photophysical Properties of the Prepared Ferric Ion Fluorescent Probe Based on Coumarin Derivatives
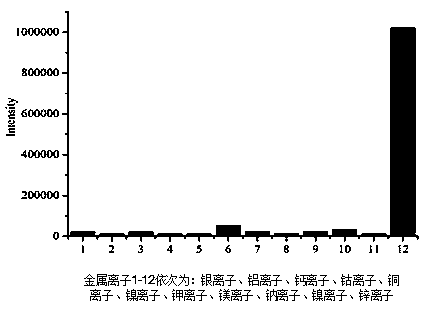
[0055] The compound has a fast response time ( image 3 As shown, the compound has a strong absorption at λ = 470 nm. When iron ions are added to the mixed solution in which the compound is dissolved, the original absorption peak at λ = 470 nm gradually decreases, and at the same time the absorption peak blue-shifts, and finally at λ = 450 nm A new absorption peak is formed, and the absorbance at 350 nm is continuously increasing. When the amount of iron ions is added to 4.2 equivalents, the absorption curve does not change, and the reaction reaches saturation. like Figure 4 As shown, with 350 nm as the excitation wavelength, the fluorescence intensity of the fluorescence peak of the compound at 416 nm gradually increased with the increase of the amount of iron ions added, and the final fluorescence intensity increased to 2 times of the original.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com